Although various techniques are currently available for preparing amorphous solid dispersions (ASD), the fundamental principles governing their formation remain essentially consistent. First, the drug’s crystalline structure is disrupted by heating or dissolving in a solvent, transitioning it into a liquid state. Subsequently, rapid cooling or drying is employed to produce an amorphous solid dispersion. Depending on the approach used to disturb the crystalline lattice, ASD preparation methods can be broadly classified into two categories: solvent-based methods and melting or fusion methods.
Solvent evaporation-based methods primarily include spray drying, electrospray, fluidized bed granulation/drying, supercritical fluid methods, spray freeze-drying, solvent casting, and others. Currently, the commercially adopted production methods mainly consist of spray drying and fluidized bed granulation/drying.
Melting-based methods mainly comprise hot-melt extrusion, KinetiSol®, microwave heating, melt granulation, ultrasonic compaction, and other variations. The currently commercialized production method within this category is predominantly hot-melt extrusion.
The preparation of amorphous solid dispersions (ASD) through solvent evaporation typically follows a two-step process: the dissolution of the drug and polymer in an organic solvent system, then followed by solvent evaporation. Rapid removal of the solent from the drug-polymer solution results in the formation of an amorphous solid dispersion comprising both the drug and polymer. Organic solvent evaporation typically takes place at temperatures below the melting point of the drug, making it more suitable for thermosensitive compounds.
In the preparation of ASD using the solvent evaporation method, the selection of a suitable solvent system is pivotal. It is not only essential to identify a solvent system capable of dissolving the drug-polymer combination and being compatible with it but also imperative to ensure that the residual solvent in the final product adheres to regulatory standards. Achieving the desired solvent properties often involve the use of a combination of multiple solvents. Prior to solvent evaporation, the chosen solvent should not adversely affect the chemical stability of the formulation components. Additionally, the use of Class I solvents should be minimized, and a preference for Class III solvents with higher safety is advisable. Table 1 provides a compilation of commonly used solvents in the ASD preparation process, including their respective boiling points and ICH classifications. In cases involving highly toxic organic solvents, thorough removal of residual solvents from the final product is necessary. Simultaneously, considering the potential impact of toxic solvents on production personnel, the implementation of proper protective measures and waste disposal practices is essential.
Solvents | Boiling point (°C) | Dielectric constant | Solubility in water (g/100 g) | Density (g/ml) | ICH limit (ppm) |
Acetone [142,143] | 56.2 | 20.7 | Miscible | 1.049 | Class 3 |
Chloroform [143] | 61.7 | 4.81 | 0.795 | 1.498 | 60 |
Methanol [144,145] | 64.6 | 32.6 | Miscible | 0.791 | 3000 |
Methylene chloride [146] | 39.8 | 9.08 | 1.32 | 1.326 | 600 |
Isopropanol [147,148] | 82.6 | 18.2 | Miscible | 0.786 | Class 3 |
Ethanol [149] | 78.5 | 24.6 | Miscible | 0.789 | Class 3 |
Dichloromethane [143,150] | 39.6 | 9.08 | 175 | 1.326 | 600 |
Dimethyl formamide [151] | 153 | 36.7 | Miscible | 0.944 | 880 |
DMSO [151] | 189 | 47 | 25.3 | 1.092 | Class 3 |
Glycerin [141] | 290 | 42.5 | Miscible | 1.261 | – |
Ethyl acetate [152] | 77 | 6 | 8.7 | 0.895 | Class 3 |
Butyl acetate [145] | 126.1 | 5.07 | 0.68 | 0.882 | Class 3 |
Water [144] | 100 | 78.5 | – | 0.998 | – |
Tetrahydrofuran [148] | 66 | 7.52 | Miscible | 0.889 | 720 |
Table 1 Organic solvents commonly employed in spray drying.
Source: T Vasconcelos, et al. Amorphous solid dispersions: Rational selection of a manufacturing process[J]. Advanced Drug Delivery Reviews, 2016,100:85-101.
If the drug is capable of melting at a temperature below 150°C without undergoing degradation, the melting-based method becomes a viable option. In this approach, a blend of the drug and polymer is heated to a molten state and promptly cooled to generate an amorphous solid dispersion (ASD). A notable advantage of the melting-based method is its capacity to circumvent the need of solvents. However, this technique may not be suitable for thermosensitive drugs, as there is a risk of drug degradation at elevated temperatures.
For the successful implementation of the melting-based method, it is essential that the drug exhibits adequate solubility and compatibility within the polymer melt. The polymer serves as a melting medium, facilitating the dissolution or dispersion of the drug and contributing to the stabilization of the amorphous form. Polymers characterized by lower melting points or glass transition temperatures (Tg) are commonly employed in this method. Widely used polymers include polyvinylpyrrolidone (PVP), vinylpyrrolidone-vinyl acetate copolymer (PVPVA), cellulose esters, acrylic esters, and poly(methyl methacrylate) derivatives. These polymers can be utilized either individually or in combination to improve amorphous stability, solubility, and bioavailability.
Plasticizers are occasionally introduced during the melting process. The melting-based ASD preparation process typically involves high shear stress, and the inclusion of plasticizers serves to diminish shear stress, decrease the viscosity of the mixture, and consequently reduce the processing temperature. Commonly employed plasticizers include d-α-tocopherol, Poloxamers (polyethylene-polypropylene-polyethylene block copolymers), low molecular weight polyethylene glycols (PEGs), and surfactants. The selection of a specific plasticizer depends on its intended function, such as whether it is to lower processing temperature or decrease melt viscosity. Traditional plasticizer additions typically range from 5%-30% (by weight), leading to significant reduction in drug loading. Additionally, residual plasticizers may impact product performance. Ideally, a plasticizer should deliver the desired plasticizing effect and be easily removable from the formulation. Often, low-boiling solvents or agents that can evaporate or sublime are chosen as plasticizers.
Beyond the solvent evaporation-based and melting-based methods discussed earlier, wet granulation and co-precipitation are additional approaches for preparation of amorphous solid dispersions (ASD). Both of these techniques have proven successful in the commercial production of ASD drugs.
Solvent Evaporation-based Method
In early-stage laboratory research, solvent casting is a viable option. For larger batch preparation, alternatives include rotary evaporation or spray drying. However, when scaling up to pilot or commercial-scale production, factors such as process efficiency, yield, particle characteristics, as well as requirements for solvent toxicity, environmental impact, operator safety, and flammability/explosion risks, must be carefully assessed. Consequently, the range of available preparation processes is restricted, with spray drying emerging as the primary choice.
Melting-based Method
In early-stage laboratory research, the preparation of melt quenching ASD can be conducted using containers in differential scanning calorimetry (DSC) or through vacuum compression molding (VCM). Larger laboratory-scale batches offer options include melt quenching with a hot plate/oil bath and hot-melt extrusion. Materials with viscosities exceeding 300 cP may pose challenges in ASD processing, and in such cases, priority may be given to hot-melt extrusion. When transitioning to pilot or commercial-scale production, the range of available preparation processes is limited, primarily centering on hot-melt extrusion.
Figure 1 illustrates the decision tree for ASD preparation methods as summarized by SV. Bhujbal, et al, providing valuable insights for contemplation. It is essential to emphasize that the selection of ASD preparation methods requires a thorough assessment of various factors, including the characteristics of the active pharmaceutical ingredient, product design and clinical requirements, the stage and strategy of product development, hardware conditions, and other relevant factors, prior to reaching a decision.
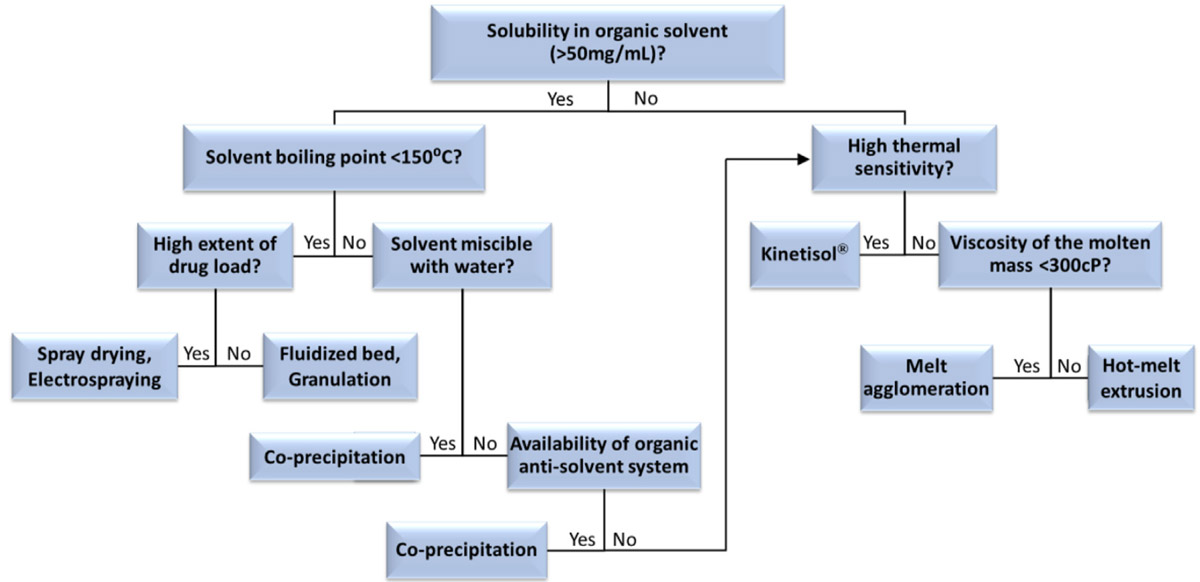
Figure 1 A method selection decision tree with the commonly used manufacturing processes for preparing ASDs.
Source: SV. Bhujbal, et al. Pharmaceutical amorphous solid dispersion: A review of manufacturing strategies[J]. Acta Pharmaceutica Sinica B, 2021,11(8):2505-2536
In addition to assessing the thermal stability and solubility of the drug, critical in selecting a manufacturing method include production batch size and the equipment availability.
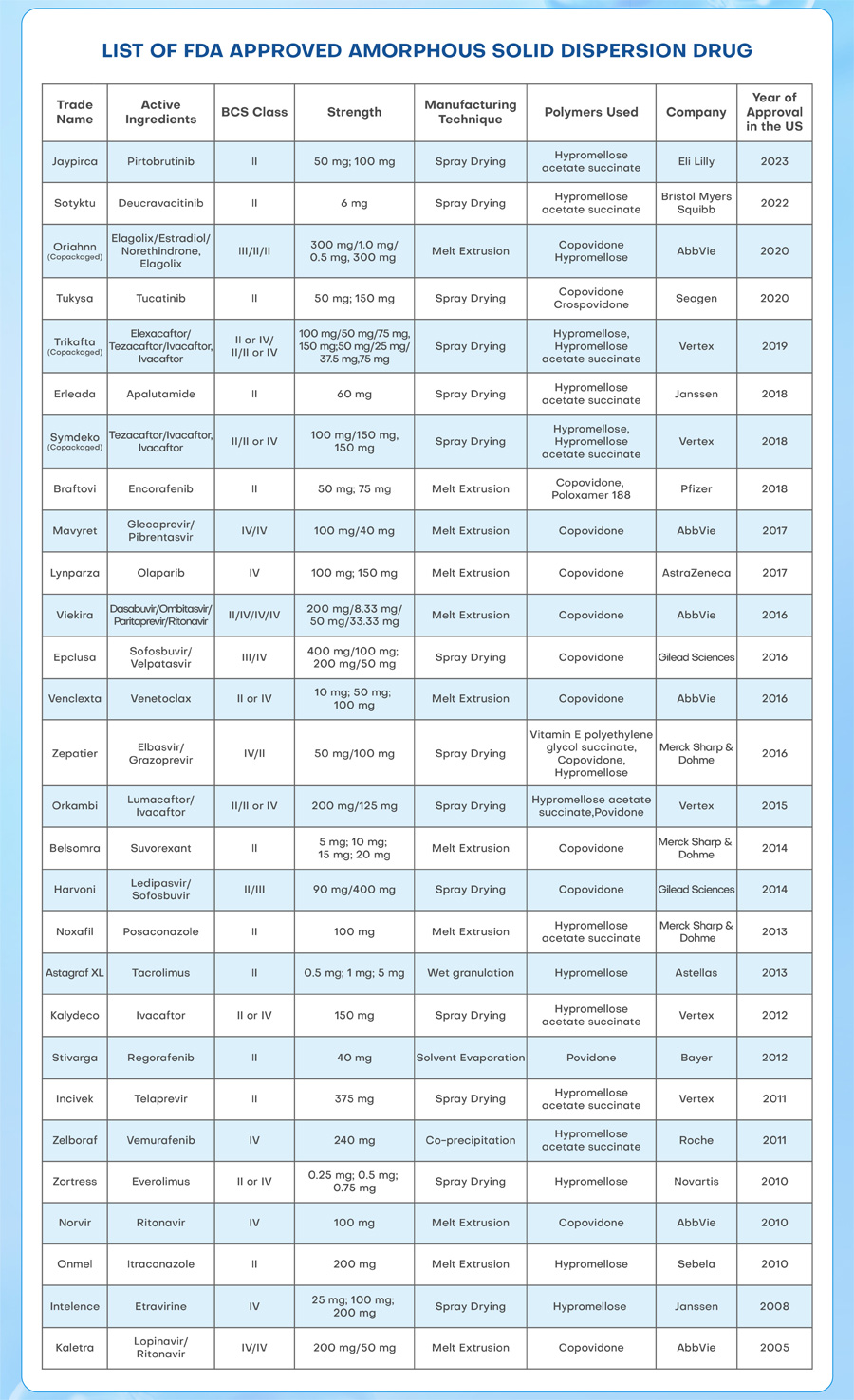
We have compiled data on a total of 28 ASD drugs approved by the FDA over the last two decades. out of these, 11 drugs were manufactured using the hot-melt extrusion method, 14 drugs were prepared using the spray drying method, and the remaining 3 drugs utilized alternative methods. It is evident that, presently, hot-melt extrusion and spray drying stand out as the most commonly used, technically advanced, and commercially implemented methods for ASD production.
1. SV. Bhujbal, et al. Pharmaceutical amorphous solid dispersion: A review of manufacturing strategies[J]. Acta Pharmaceutica Sinica B, 2021,11(8):2505-2536.
2. T Vasconcelos, et al. Amorphous solid dispersions: Rational selection of a manufacturing process[J]. Advanced Drug Delivery Reviews, 2016,100:85-101.