In recent years, the utilization of Amorphous Solid Dispersions (ASD) has gained increasing interest, particularly for enhancing the solubility of drugs with inherently low solubility. Within ASDs, drug molecules exist in a high-energy amorphous state, predisposing them to a spontaneous proclivity towards transitioning into a more stable, lower-energy crystalline form. This phenomenon poses critical challenges, including phase separation and recrystallization during storage, which in turn can adversely affect the physical stability, efficacy, and bioavailability of the ASD. Ensuring sustained stability over the entire shelf life thus emerges as a paramount objective in the development of ASD formulations. This paper aims to elucidate a range of pivotal factors that significantly influence the physical stability of ASDs.
In the development of a robust and effective Amorphous Solid Dispersion (ASD) formulation, it is imperative to rigorously evaluate several key factors throughout the polymer screening phase and the selection of preparative methodologies. These critical factors encompass drug-polymer miscibility, drug solubility in the polymer, drug-polymer interactions, manufacturing processes, and storage conditions. The strategic consideration of these elements is fundamental to achieving the desired stability and functionality of the ASD formulation.
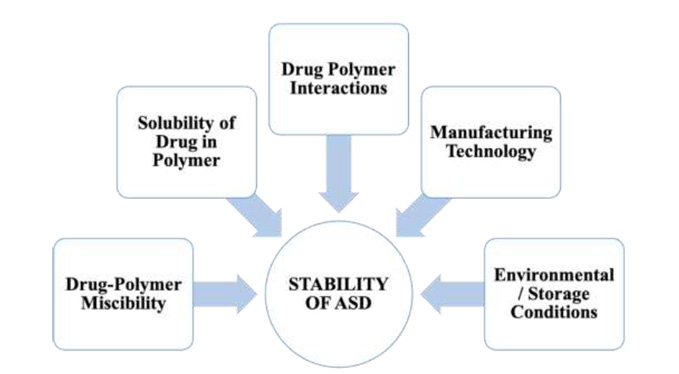
Figure 1. A detailed overview of various critical factors that affect the stability of ASD.
Drug-polymer miscibility and drug solubility in the polymer are essential factors in the formulation of Amorphous Solid Dispersions (ASD). ASD, being a homogeneous system, requires a high degree of miscibility between the drug and polymer. Enhanced miscibility and solubility directly correlate with increased drug load capacity in the ASD. Stability of the ASD is maintained when the drug concentration within the carrier remains below its solubility limit at the storage temperature. In contrast, a state of supersaturation of the drug in the carrier elevates the chemical energy, predisposing the drug molecules to crystallization, which compromises the stability. Therefore, the strategic selection of polymers that exhibit high miscibility is vital for the development of stable ASD formulations with optimal drug loading capacity.
The dynamics between drugs and polymers are fundamental to the physical stability of Amorphous Solid Dispersions (ASD). These interactions bolster stability via two primary mechanisms. The first mechanism involves interactions like hydrogen bonding and ionic interactions between drugs and polymers, which serve to decrease the molecular mobility of drugs within the ASD, consequently augmenting its physical stability. Typically, the strength of these drug-polymer interactions is directly proportional to the enhanced stability. The second mechanism pertains to how drug-polymer interactions impact drug-polymer miscibility and the solubility in the polymer, both of which are critical factors influencing the overall stability of ASD.
The methodologies and procedures employed in the preparation of Amorphous Solid Dispersions (ASD) are pivotal in determining their stability. ASDs prepared using different techniques exhibit notable variances in physical stability. These differences arise from alterations in molecular interactions and physicochemical properties, leading to varied drug dispersion, microphysical structure and drug lattice destruction. The most prevalent techniques for ASD preparation are hot melt extrusion and spray drying. ASDs produced via hot melt extrusion typically exhibit a lower specific surface area and higher bulk density. Conversely, ASDs prepared by spray drying tend to have a lower bulk density and a higher specific surface area. ASDs with greater specific surface area are more susceptible to moisture absorption, potentially increasing the moisture content and reducing the glass transition temperature of the ASD, thereby heightening the risk of recrystallization. Therefore, it is especially critical for spray-dried ASDs to be stored under conditions that mitigate moisture absorption and prevent recrystallization.
The glass transition temperature (Tg) is a critical parameter influencing the stability of Amorphous Solid Dispersions (ASD). Being a single-phase system, as ASD possesses a distinct Tg. Storage temperature surpassing this Tg can lead to increased molecular mobility and a heightened propensity for crystallization. It is thus essential to store ASDs at temperatures significantly lower their Tg. Given that most carriers used in ASDs are hydrophilic and exhibit strong hygroscopic properties, the moisture they absorb can function as plasticizer, reducing the Tg of the system. To mitigate this, it is crucial for ASDs to be shielded from environmental moisture. Opting for packaging materials with excellent moisture barrier qualities and implementing sealed storage at low temperatures are practical strategies to preserve the physical stability of ASDs.
While Amorphous Solid Dispersion (ASD) has emerged as a favored approach for enhancing the solubility and bioavailability of drugs with low solubility, addressing stability remains a primary challenge. Extensive research and various successful instances have demonstrated that stability and quality of ASD formulations can be effectively maintained. This is achievable through the careful selection of appropriate polymers, the design of rational formulations, the use of suitable preparations and the stringent control of storage conditions.
References:
[1] Konda KK, Dhoppalapudi S. A Review of Various Manufacturing Approaches for Developing Amorphous Solid Dispersions[J]. Journal of Drug Delivery & Therapeutics, 2022, 12(6):189-200.
[2] Jelic, D. Thermal Stability of Amorphous Solid Dispersions[J]. Molecules, 2021, 26(1):238-255.
[3] Lin et al. Physical Stability of Amorphous Solid Dispersions: A Physicochemical Perspective with Thermodynamic, Kinetic and Environmental Aspects[J]. Pharmaceutical Research, 2018, 35(6):125-142.
[4] 刘旭等. 固体分散体物理稳定性影响因素及抗老化研究进展[J]. 中国现代应用药学, 2011,28(8):710-716.
[5] 欧丽泉等. 聚合物载体对固体分散体稳定性影响的研究进展[J]. 中国医院药学杂志, 2020, 40(8): 2077-2081.