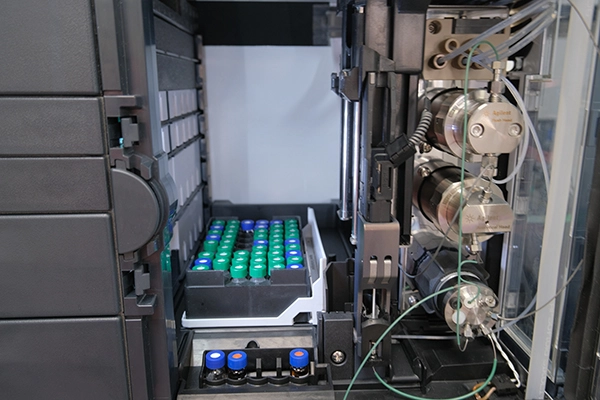
Biologics CMC Analytical Lab, operates a cutting-edge 5,300 sq.ft lab in New Jersey. We provide advanced analytical services for biotech and pharma companies developing biologics. Our expertise spans a broad range of therapeutic modalities including antibodies, bi/multi-specifics, proteins, ADCs, mRNA, LNP, and nucleotides, supporting projects from discovery to the clinic.
Biologics are being used as drugs to treat a variety of human and animal diseases including autoimmune diseases such as rheumatoid arthritis. In the case of autoimmune diseases, some biologics interrupt signals, and pathways in the immune system to reduce the damage inflicted by the autoimmune conditions. Biologics can be very micro-heterogenous mixtures of structural variants that are difficult to identify and characterize. Biological products are generally heat sensitive and susceptible to microbial contamination.



.png)