Crystal Pharmatech provides a one-stop Clinical Supply Service from pre-clinical to post-marketing.
| One-Stop Clinical Supply Service |
|---|
| Packaging Design; Label Design; Translation; Randomization; Comparator Sourcing;
| Global Transportation; Timely Monitoring; Cold Chain capability; Importation; Exportation;
| |
Primary and Secondary Package
|
|---|
Primary Packaging | GMP environment: | 

| 
|
Secondary Packaging | | Package and Label design: Labels design: Single panel, two panels, wrap around, booklet labels Package design: Wallet card Others: Outer carton and temper seals
| 
|
| Storage, distribution, return and destruction |
|---|
| Storage | Distribution | Return | Destruction |
Classified storage management by category, temperature zone, and batch number; Provide cycle counting and validity management services; Comply with national regulatory requirements; IRT integration services.
| Temperature controlled logistics services - local and global; Import and export requirements; Documents for distribution can be provided, eg. COA, data logger calibration certificate and POD; Site to site transfer service; Direct to patient service.
| Return report to the customer; Provide return inventory services; Different levels of return reconciliation: kit level and shipper box level.
| |
| Covering China Mainland, China Taiwan, Australia, New Zealand, Europe, the United States and Canada |
International transportation
Importation & Exportation
Import permit/CIQ application
Custom clearance
Cold chain Global network
Value Added Service
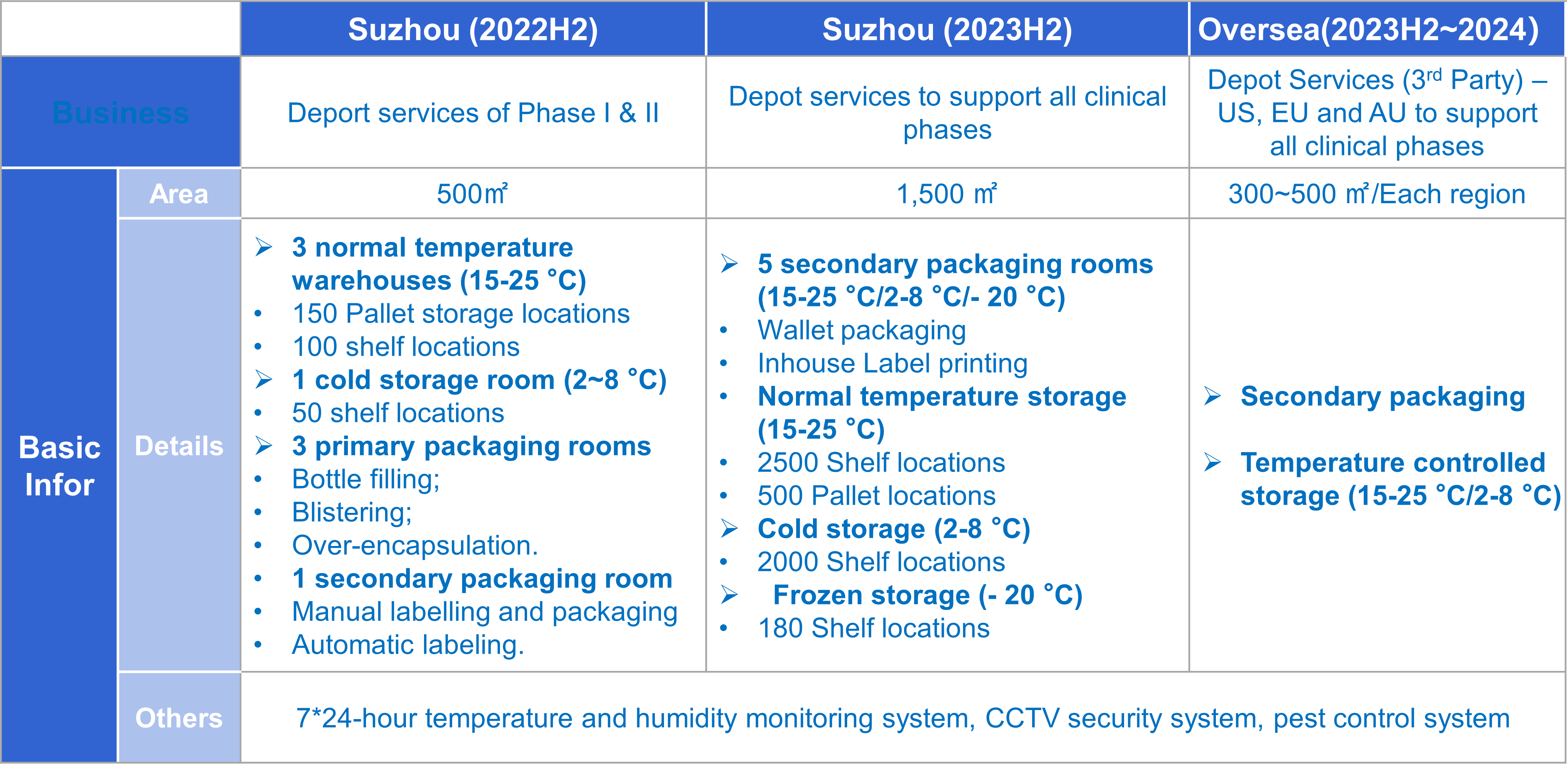
Capacity
By providing your e-mail address, you agree to receive an e-mail response from Crystal Pharmatech to your inquiry. The information you submit will be governed by our Privacy Policy.



