11 Apr 2025
Nuclear Magnetic Resonance (NMR) Spectroscopy is a ubiquitous analytical technique utilized across a wide range of disciplines. In the pharmaceutical industry, NMR is used in drug discovery and development to verify structures, to monitor reactions, and to characterize the solid phase structure of APIs and drug products.
In the solid-state, NMR is sensitive to inter- and intra-molecular interactions. Specific NMR techniques allow the unambiguous determination of molecular structure, orientation, and dynamics. In principle, a series of solid-state NMR (ssNMR) spectra can provide much of the information necessary to completely characterize the solid-phase nature of a sample including:
Salt Disproportionation
Polymorphs Structure and Ratios
Solvates and Hydrates Locations
Amorphous and Crystalline Dispersions
NMR Active Nuclei
In solution, proton (H-1) NMR is, by far, the most common nuclei observed. In solids, carbon NMR (C-13) spectra can be simplified using techniques such as Magic Angle Spinning (MAS) and High Power Decoupling (HDEC) to simplify complex spectra and to extract the abundance of information available (Figure 1).
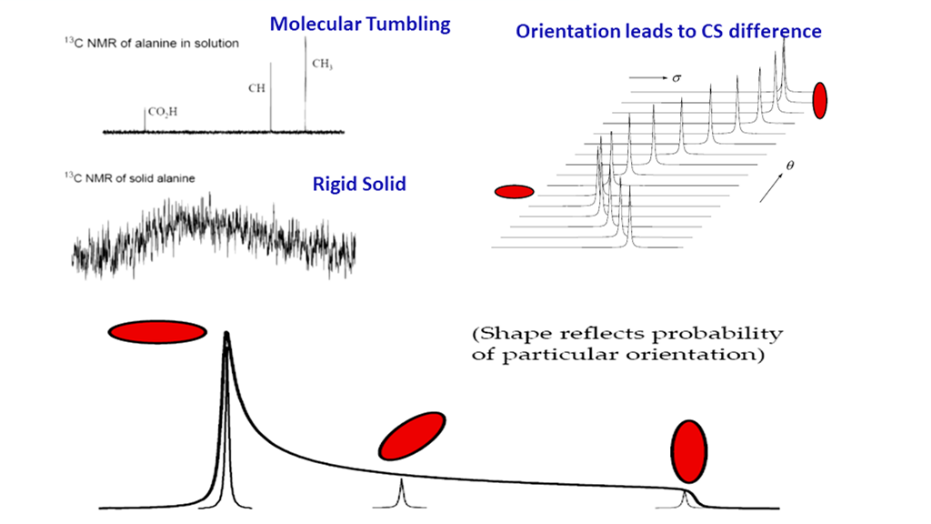
Figure 1: NMR spectra under a variety of experimental conditions. Line-narrowing techniques are used to achieve liquid-like spectra from solids.
For molecules containing fluorine (F-19), ssNMR has unique capabilities. F-19 is an “NMR friendly” nucleus: 100% natural abundant, spin 1/2, and a wide chemical shift range. F-19 ssNMR is able to differentiate crystalline polymorphs, and distinguish amorphous and crystalline components in a lattice. Recently, phosphorus (P-31) has also been used in solid phase characterization (Figure 2).
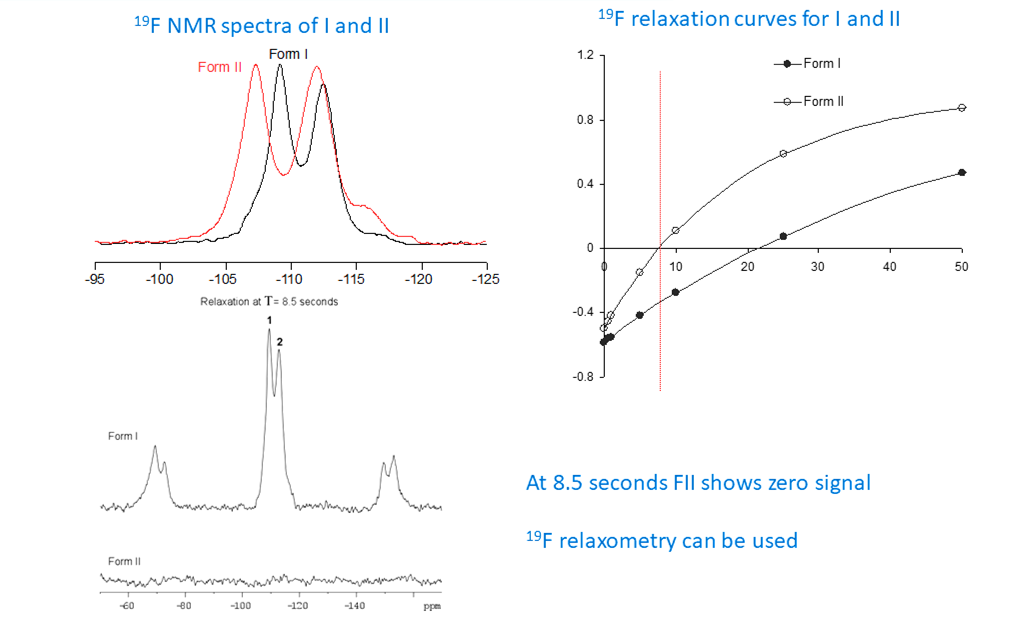
Figure 2: Fluorine-19 data differentiating two forms of an API utilizing chemical shift and relaxation information.
Sample Quantity
Sample quantity is largely driven by the rotor's size (the sample container used inside the spectrometer). Several rotor sizes are available, requiring sample quantities from a 10's of milligrams to a few hundred milligrams. The choice of rotor and sample quantity is a matrix of sensitivity. The amount of sample available, loading (in the case of drug product) and NMR parameters are required to achieve the desired results (i.e. spin speed, decoupling strength, and relaxation times). NMR is a quantitative technique with a unique advantage of having uniform signal response across resonances. Limits-of-detection (LOD) and limits-of-quantitation (LOQ) can vary widely depending upon the characteristics of the sample, instrumentation, and the specific NMR experiment under consideration. NMR is also a signal-averaging technique, whereby longer experiment times can improve signal-to-noise which directly impacts LOD and LOQ.
1-D, 2-D and Relaxation
Most NMR spectra are considered 1-dimensional (1-D), which is represented by the plot of the chemical shift vs signal intensity. The position of the peaks is directly related to their chemical environment. In addition, the line widths observed can provide valuable information about the motional characteristics of the sample including exchange between crystalline forms, and/or conversion of crystalline to amorphous form (Figure 2).
Multi-dimensional NMR varies NMR parameters in a series of acquisitions to create a map of resonances indicating connectivity between atoms. Countless varieties of 2-dimensional (2-D) techniques have been invented to measure specific inter-molecular and intra-molecular interactions. NMR can correlate distinct resonances from one part of molecule with resonances from another part of the same molecule. In a lattice with multiple forms, 2D NMR can show connectivity between atoms on the same molecule or between two different molecules, e.g. between excipient and API (Figure 3).
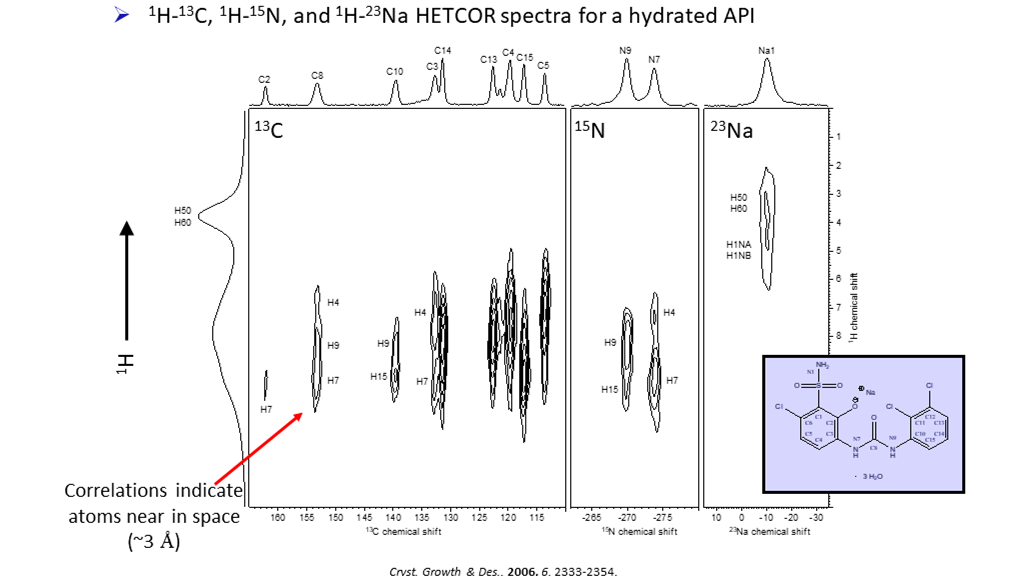
Figure 3: Heteronuclear correlation experiments illustrating connectivities between different nuclei.
Relaxation is a phenomenon of the magnetic resonance technique that depends largely on the molecular motion of the species being measured and is sensitive to the lattice (T1 relaxation) or to other neighboring atoms (T2 relaxation). Various experimental methods can capitalize on the sensitivity of relaxation to molecular motion. Both T1 and T2 offer a mechanism to measure solid-state characteristics of intermediates, APIs, drug products, and excipients.
Conclusions
Largely due to its unique capabilities, ssNMR spectroscopy of pharmaceutical materials has evolved from a sparingly used technique into an important component of pharmaceutical development. Continued innovation in NMR technologies provide new and accessible methods for obtaining detailed information regarding the inter-molecular and intra-molecular structure and dynamics of solid-phase forms of APIs and formulations from early stage discovery through marketed Drug Products.


