11 Apr 2025
Crystal Pharmatech are a specialized CRO/CDMO focused on Solid State Research, Pre-Formulation, Formulation Development and Manufacturing. Our strength is our focus and expertise in these specialties and the outcome for the client is the following Mol2Med™ Integrated Services: First-Time-Right 3-STEP Approach, designed to expedite small molecule lead compounds or preclinical candidates into Phase I and beyond, with unparalleled efficiency and precision. This First-Time-Right 3-step approach begins at the lead optimization/PCC stage: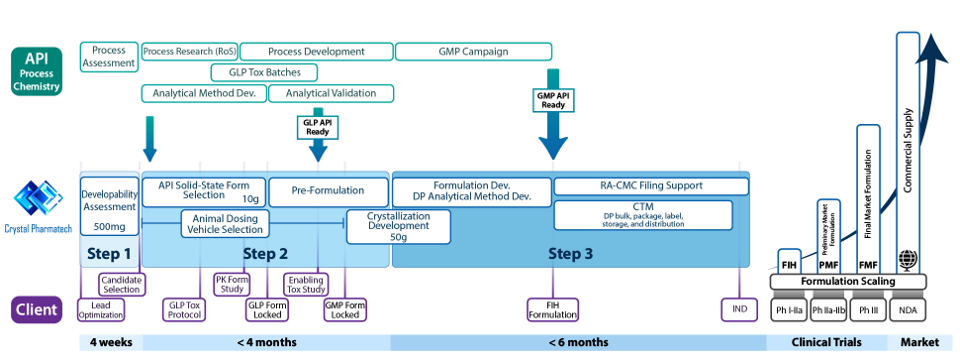
Step 1: Developability Assessment
Our approach determines the physicochemical properties of all lead candidates to find the most “developable” lead, benchmarking against a series of industry standards and providing a high-level approach for achieving the most probable success in GLP Tox and FIH. For a given preclinical candidate (PCC), this will also help to determine whether a free form or salt, and a conventional formulation using crystalline form or amorphous solid dispersion should be selected for further development.
Step 2: Solid Form Screening/Selection and Pre-Formulation
Our approach begins with comprehensive screening and selection of the optimal crystalline form for the API. Subsequently, we develop an optimal preclinical formulation to support PK/PD and GLP Tox animal studies.
Step 3: Formulation Development and CTM Manufacturing
API-specific "First-time-right" strategy that yields the best formulation for Phase I and subsequent Clinical Studies. This allows Pre-Market Formulation and Final-Market Formulation late-stage development to focus on process optimization and scale up, without the need for significant formulation change and hence the need for human PK bridging studies.
Contrast this to the traditional “fit-for-purpose” method (for example, powder in a capsule or prototype capsule or tablet), which requires human PK bridging studies after significant re-formulation. This results in significant additional cost and time.
This innovative approach guarantees a robust API form and a scalable manufacturing process, culminating in a First-Time-Right formulation for Phase I. Streamlining the transition to future clinical studies upon Phase I success, our First-Time-Right approach sets the foundation for optimized drug development and success beyond.


