11 Apr 2025
Crystal Pharmatech is recognized as a reliable partner by providing high-quality, fast, flexible clinical and commercial production services to meet our customers' different needs, helping them accelerate their drug launches.
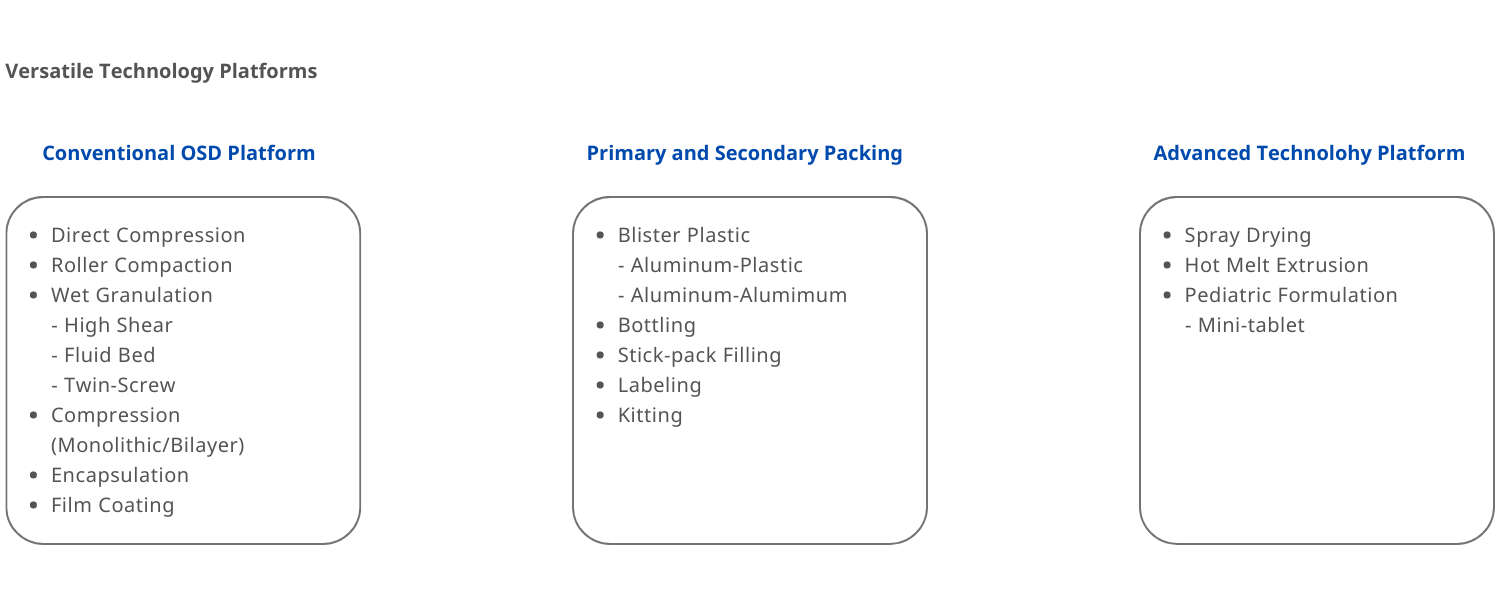
Dosage forms
Solid oral dosage forms (tablet, capsule, sachet)
Amorphous Solid Dispersions (ASD, Spray Drying & Hot Melt Extrusion) for insoluble compounds & PROTAC & oral peptides
Pediatric Dosage Form (mini-tablet)
GMP manufacturing facilities
Suzhou, China: our state-of-the-art cGMP clinical and commercial manufacturing plant is located at Suzhou Industry Park,45 miles (~70 kilometers) from Shanghai, performing pilot and production runs at a range of scales up to 300kg as well as small-scale manufacturing.
Capacity and capability:
- Current: 0.5-120kg, OEB 3 and below
- Upcoming: 30-500kg, OEB 1-5
Toronto, Canada: A GMP facility in Toronto is under construction, enabling formulation development and manufacturing services from both Suzhou and Toronto sites.
An experienced team with highly efficient execution
The GMP team are experts with extensive industry experience, unique skills in optimizing and troubleshooting production and analysis, and broader chemistry expertise. We ensure that your project gets the focus it needs and with our expertise in technical transfer, we can increase manufacturing efficiency.
World-class manufacturing equipment
Taking advantage of our advanced technologies and world-class manufacturing equipment, Cyrstal Pharmatech is uniquely positioned to deliver high-quality drug products to achieve or beyond your goals.
cGMP and QMS meeting NMPA, FDA, and EMA requirements
Crystal Pharmatech’s infrastructure, quality systems, and verification and validation procedures comply with international cGMP standards, laying a solid foundation for us to provide CDMO services that meet the FDA, NMPA, and EMA regulations.