07 Nov 2023
How Crystal Pharmatech, a leading CRDMO, leverages GastroPlus® to guide small molecule development and manufacturing decisions.
GastroPlus® Background
GastroPlus® is an advanced software tool used in pharmaceutical development for physiologically-based pharmacokinetic (PBPK) modeling.
GastroPlus® leverages in-vivo and in-vitro data to simulate and predict the ADME profile of drug candidates in animal and human models.
It simulates partitioning and absorption for 9 different segments of the GI tract.
Crystal Pharmatech and GastroPlus®
1. First, we gather all available data, including:
Physicochemical properties
Dose, dosage form, dose frequency
IV data
PO data
2. Then, we build the animal models.
Animal models are consistently updated as new PK data becomes available
Species-specific modeling accounts for differences in GIT, metabolism, and more
3. Next, we leverage the data and models built in GastroPlus® to generate human PK predictions including:
Human-specific bioavailability and PK
Impact on F% from varying doses and dosing frequency
4. Finally, we analyze the parameter sensitivity to understand what we can do to maximize in vivo performance.
Human-specific bioavailability and PK
Impact on F% from varying doses and dosing frequency
Outcomes:
GastroPlus®, combined with experienced solid-state chemists and formulators, can provide invaluable insights for lead candidate selection and the optimal GLP Tox and FIH formulation approaches.
The insights gained by integrating GastroPlus® into your research and development strategy save drug innovators time, money, and material.
Crystal Pharmatech background
To be pulled from previous flyers…
An innovator with a first-in-class drug is heading into GLP Tox. Toxicity is difficult to obtain and the FIH dose is uncertain, so the client set a high target exposure in GLP Tox.
What dose is needed to reach the high target exposure in GLP Tox and enable flexibility in FIH dose escalation?
Based on the dose, determined with the help of PBPK modeling, what is the best formulation to proceed with?
Modeling Enterohepatic Recirculation (EHC) in Dogs
The dog model was preferred over the rat model as rats do not have a gallbladder.
Model guided by oral data to determine the amount of EHC.
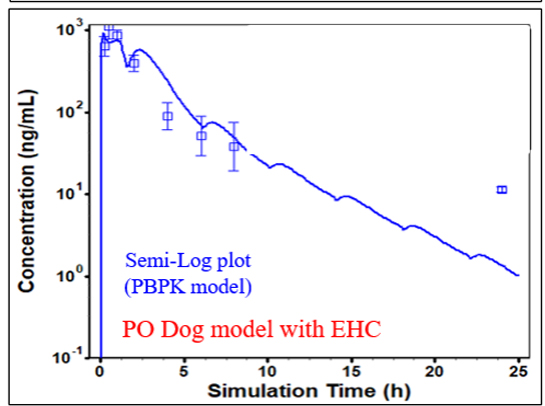
Predicted dog clearance was used to scale into human model.
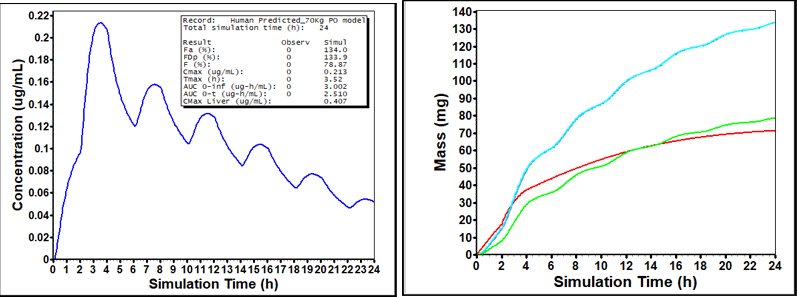
Human model shows high fraction absorbed driven by EHC with strong bioavailability
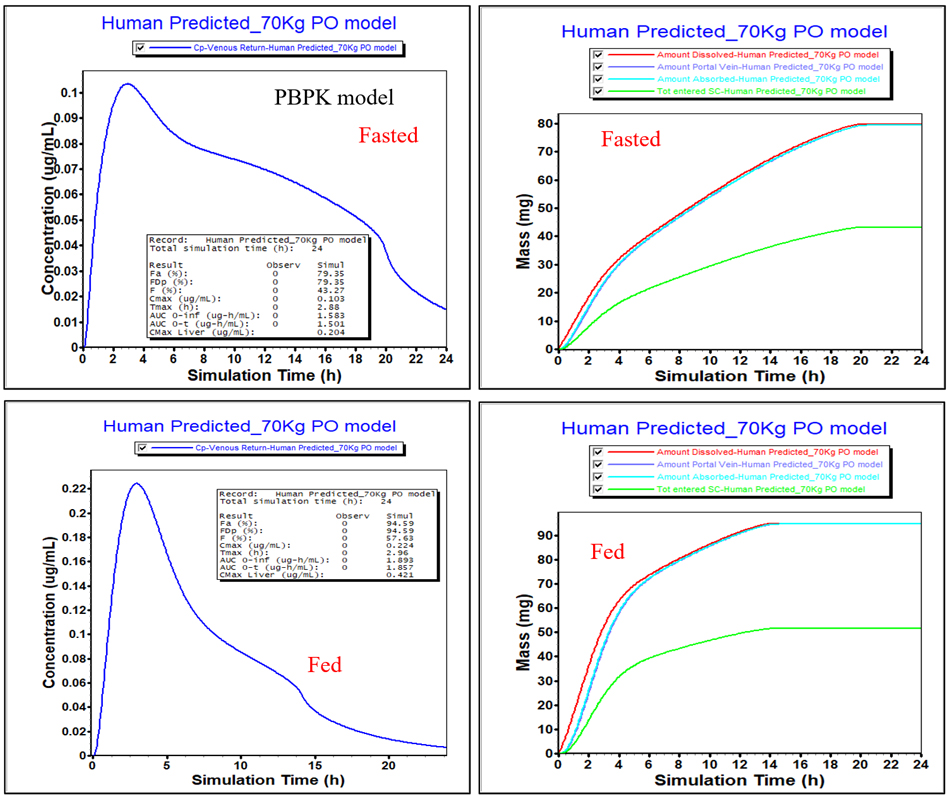
Due to uncertainty in the amount of EHC, the lower limit without EHC was also generated to establish a “worst case scenario”.
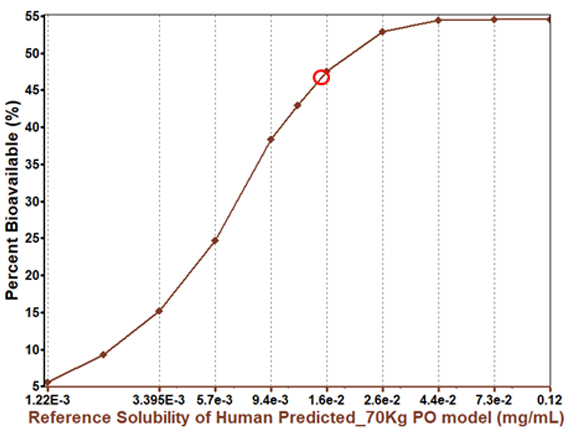
Parameter sensitivity showed increased solubility will improve bioavailability, but by a limited amount.
This indicated the bioavailability may not be entirely solubility limited.
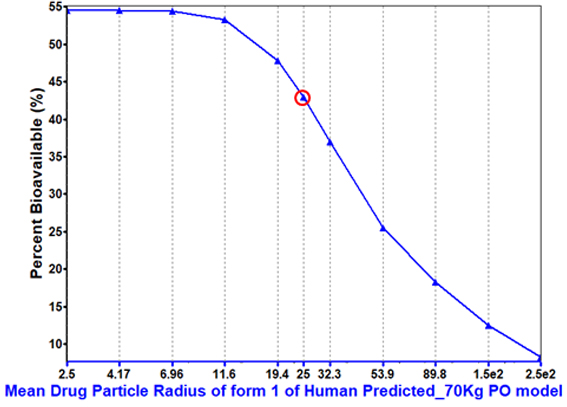
Particle size is predicted to have an impact on bioavailability.
This dictated the particle size range that was adequate to achieve target.
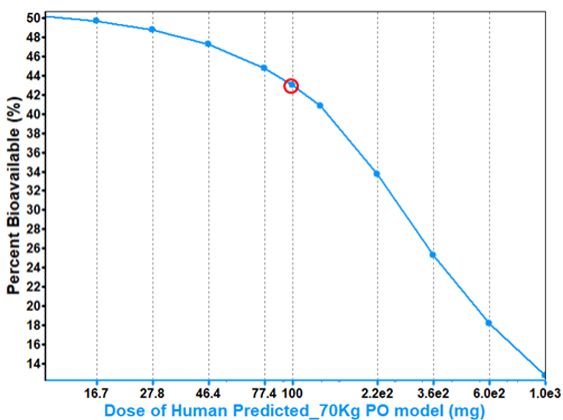
Increasing dose is predicted to have an impact on bioavailability.
Solubility limited drug and increasing dose increase precipitation potential, decreasing bioavailability.
Outcome:
GastroPlus® provided the client and formulation team with critical insights to guide dosing and formulation development strategy, maximizing bioavailability and enabling the greatest flexibility in FIH dose escalation.
Dose strength reduced from initial estimates to prevent precipitation
Solubility enhanced with amorphous formulation
Particle size controlled to maximize the predicted impact
