Hot melt extrusion (HME) technology is widely used in the development of amorphous solid dispersions, offering advantages such as a solvent-free preparation process, continuous operation, high automation control, reliable reproducibility, and scalability. Polymers play essential roles in the preparation of polymer-drug amorphous solid dispersions. Their functions include stabilizing structural integrity, enhancing solubility, regulating compatibility, and preventing phase separation, all of which critically impact the physical stability and in vitro performance of amorphous drugs. This paper explores the criteria for selecting suitable polymers for the preparation of amorphous solid dispersions using hot melt extrusion.
The specialized nature of the hot melt extrusion process means that the rheological properties of drug-polymer melt during extrusion are intricate, necessitating precise control of the thermodynamic and rheological properties of the polymers employed. Polymers that are suitable for hot melt extrusion processing must meet the following criteria:
1. Thermoplasticity: Polymers must exhibit thermoplastic characteristics, which entails the ability to soften and deform upon heating. This characteristic is essential as it facilitates the processing and molding of the polymers through the extrusion die of the extruder.
2. Glass Transition Temperature (Tg): The Tg of the polymer should align with the temperature range required for the extrusion process. Polymers with Tg between 50-180°C are generally more suitable for this purpose. Additionally, the Tg of the polymer can be modified through the incorporation of suitable plasticizers if adjustments are necessary.
3. Thermal Stability: Polymers used in the extrusion process must maintain structural integrity and not decompose or degrade at operating temperatures. It is crucial to confirm the polymer’s stability throughout the extrusion to prevent the formation of potentially toxic decomposition products.
4. Melt Viscosity: Polymers with medium to low melt viscosity are preferable for facilitating the extrusion process. Typically, polymers exhibit pseudoplastic behavior, where their viscosity decreases with increasing shear stress. This characteristic enhances the flowability and processability of the polymer within the extruder.
5. Hygroscopicity: Selecting polymers with low hygroscopicity can minimize the impact of moisture on the stability of pharmaceutical amorphous solid dispersions. While the high temperatures in hot melt extrusion help the evaporation of adsorbed moisture, any water absorbed post-extrusion can lower the Tg and increase the risk of recrystallization due to plasticizing effects.
6. Solubility: The solubility and compatibility of polymers with APIs are critical for determining the drug loading capacity. Choosing polymers that exhibit good solubility ensures effective dispersion and dissolution of drugs within the polymer matrix. The solubility properties of polymers are influenced by the chemical structure of the monomers, particularly the presence of lipophilic groups and hydrogen bond donors or acceptors.
7. Regulatory Compliance: Given the extensive incorporation of polymers in final drug formulations, it is critical to ensure that they adhere to applicable regulatory requirements. Choose non-toxic polymers that comply with human use standards and ensure they are approved for use in pharmaceutical applications.
When a single polymer does not fulfill all requirements, combining different polymers may may be considered to improve the overall properties of the formulation.
The following are several common polymers suitable for hot melt extrusion.
Polyvinylpyrrolidone (PVP), also referred to as Povidone, is a polymer synthesized through the polymerization of N-vinyl-2-pyrrolidone. The molecular structure of PVP includes amide bonds, which facilitate the formation of intermolecular hydrogen bonds with drugs, acting as hydrogen bond acceptor groups. This interaction enhances drug wettability and helps to inhibit drug crystallization. However, higher molecular weight PVPs generally possess high glass transition temperatures (Tg), rendering them less suitable for preparing solid dispersions via hot melt extrusion.
PVP/VA (Polyvinylpyrrolidone/Vinyl Acetate Copolymer), commonly known as Copovidone, is a copolymer composed of vinylpyrrolidone and vinyl acetate. The inclusion of the flexible monomer vinyl acetate enhances the plasticity of the polymer and lowers its glass transition temperature (Tg). These modifications expand the processing temperature range and improve the rheological properties of PVP/VA, making it a preferred polymer for various pharmaceutical applications, with a broader utility scope compared to PVP alone.
Soluplus® is a copolymer designed for hot melt extrusion (HME) and solid dispersion technologies. It is synthesized through copolymerization reactions that graft hydrophobic vinyl caprolactam monomers and vinyl acetate monomers onto hydrophilic polyethylene glycol (PEG) main chains. This structure imparts distinct amphiphilic characteristics to Soluplus®, enabling it to form micelles in water and significantly enhance dissolution efficiency. Furthermore, the inclusion of vinyl acetate and PEG in its composition lowers the glass transition temperature (Tg) of the polymer. This reduction in Tg allows Soluplus® to be processed at lower temperatures, making it particularly advantageous for the formulation of heat-sensitive drugs.
Hydroxypropyl Methylcellulose (HPMC) is a non-ionic cellulose ether and a semi-synthetic, inert, viscoelastic polymer. It belongs to the category of mixed ethers derived from cellulose.
Hydroxypropyl Methylcellulose Acetate Succinate (HPMCAS) is a copolymer composed of hydroxypropyl methylcellulose acetate and succinate, exhibiting amphiphilic properties. The copolymer is classified into three types—L, M, and H—based on the degrees of acetylation and succinylation. The L type, which has the highest succinyl content, exhibits the strongest hydrophilicity, whereas the H type, with the highest acetyl content, shows the strongest hydrophobicity. These variations significantly influence drug release behavior and crystallization inhibition across different pH levels. Specifically, an increase in hydrophobic acetyl content can enhance adsorption onto the drug crystal surface, thereby preventing contact with the surrounding medium, enhancing crystallization inhibition, and decelerating drug release. Consequently, the L type, compared to the M and H types, facilitates more rapid drug release in the intestinal environment. Both HPMCAS and Hydroxypropyl Methylcellulose (HPMC) are favored excipients for the preparation of amorphous solid dispersions using hot melt extrusion (HME).
Polyethylene glycol (PEG) is widely used as a carrier material in the preparation of solid dispersions for poorly soluble drugs. Its favorable attributes, such as excellent water solubility, safety profile, low melting points in medium and high molecular weight forms, and robust stability, make it particularly suitable for use in hot melt extrusion technology. Furthermore, PEG can be combined with other polymers as plasticizers, enhancing the solubility and wettability of poorly soluble drugs, thereby improving their bioavailability.
Eudragit® represents a widely used family of polymethacrylate polymers, classified into gastric, intestinal, and permeable types based on functional group differences. These polymers can be used either alone or in combination with other polymers to tailor drug release profiles in pharmaceutical formulations, facilitating immediate, sustained, or pulsatile release. Specifically, Eudragit® EPO is favored in hot melt extrusion (HME) processes due to its effectiveness in enhancing the dissolution of poorly soluble drugs. Eudragit® EPO is a pH-sensitive, water-soluble polymer that dissolves in environments with a pH of less than 5 and swells in those with a pH greater than 5. It has a glass transition temperature (Tg) of approximately 50°C and exhibits suitable thermoplastic properties, making it ideal for the preparation of amorphous solid dispersions.
Table 1 details the amorphous solid dispersion drugs developed through the hot melt extrusion process that have been approved by the US FDA over the past 20 years. Among the polymers utilized, PVP/VA (Polyvinylpyrrolidone/Vinyl Acetate Copolymer) has been the most widely employed.
Trade name | Active Ingredients | Strength | Polymer | Innovator | Approval date |
Oriahnn | Elagolix/Estradiol/Norethindrone, Elagolix | 300 mg/1.0 mg/0.5 mg, 300 mg | PVP/VA, HPMC | AbbVie | 2020 |
Braftovi | Encorafenib | 50 mg; 75 mg | PVP/VA, Poloxamer 188 | Pfizer | 2018 |
Mavyret | Glecaprevir/Pibrentasvir | 100 mg/40 mg | PVP/VA | AbbVie | 2017 |
Lynparza | Olaparib | 100 mg; 150 mg | PVP/VA | AstraZeneca | 2017 |
Viekira | Dasabuvir/Ombitasvir/ Paritaprevir/Ritonavir | 200 mg/8.33 mg/50 mg/33.33 mg | PVP/VA | AbbVie | 2016 |
Venclexta | Venetoclax | 10 mg; 50 mg; 100 mg | PVP/VA | AbbVie | 2016 |
Belsomra | Suvorexant | 5 mg; 10 mg; 15 mg; 20 mg | PVP/VA | Merck | 2014 |
Noxafil | Posaconazole | 100 mg | HPMCAS | Merck | 2013 |
Norvir | Ritonavir | 100 mg | PVP/VA | AbbVie | 2010 |
Onmel | Itraconazole | 200 mg | HPMC | Sebela | 2010 |
Kaletra | Lopinavir/Ritonavir | 200 mg/50 mg | PVP/VA | AbbVie | 2005 |
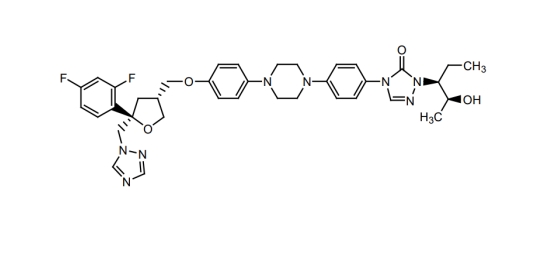
Posaconazole Chemical Structure
Posaconazole is a second-generation triazole antifungal agent extensively utilized for the prevention and treatment of invasive fungal infections. Merck has developed three FDA-approved dosage forms of posaconazole: oral suspension, enteric-coated tablets, and injections. The initial approval of posaconazole in the United States occurred in 2006, with the oral suspension of crystalline posaconazole as the first approved dosage form. Subsequently, delayed-release tablets and injections were approved in 2013 and 2014, respectively.
It is a weakly alkaline and exhibits poor solubility, which decreases sharply as the pH of the medium increases, falling to less than 1 μg/mL in environments with a pH greater than 5. The higher pH levels in the intestines substantially limit its absorption in these regions. Consequently, to enhance the absorption of posaconazole oral suspension into the bloodstream, it is recommended that patients consume it alongside a high-fat meal or nutritional supplements, which can help extend the gastric retention time.
Posaconazole delayed-release tablets utilize pH-dependent HPMCAS as a carrier and employ hot-melt extrusion to prepare an amorphous solid dispersion, which enhances bioavailability directly without the need for a high-fat diet. The M-type HPMCAS used as the carrier effectively inhibits posaconazole recrystallization and maintains it in a saturated state for an extended period. In vivo studies indicate that posaconazole delayed-release tablets not only significantly enhance bioavailability but also minimize the impact of dietary variations on posaconazole blood concentrations. For instance, when administered at a dose of 100 mg under fasting conditions, the delayed-release tablets achieve a plasma concentration approximately four times higher than that of the oral suspension. Furthermore, the plasma concentrations of the delayed-release tablets remain consistent under both fasting and fed conditions, whereas the plasma concentration of the oral suspension is about twice as high when taken with food compared to fasting. The enteric solubility properties of HPMCAS prevent posaconazole dissolution in the stomach, thus mitigating the effects of gastric contents on the drug and avoiding recrystallization. Additionally, the succinyl groups within HPMCAS can form ionic interactions with posaconazole, further enhancing its dissolution and absorption in the intestines and significantly improving both the plasma concentration and overall bioavailability of the drug.
Selecting the appropriate polymer is a critical factor in the successful development of amorphous solid dispersion formulations. At present, the variety of polymers suitable for commercial production via hot-melt extrusion is limited. However, it is anticipated that continued advancements in polymer research will expand the applications of hot-melt extrusion technology in developing amorphous solid dispersion formulations, broadening their potential and efficacy in pharmaceutical manufacturing.
[1] Wikipedia
[2] US FDA official website
[3] Patent CN102065842A
[4] 李凌晖等.热熔挤出工艺中促进难溶性药物溶出的聚合物载体概述. 中国医药工业杂志, 2021, 52(2): 180-189
[5] Kyriakos Kachrimanis et al. Polymers as Formulation Excipients for Hot-Melt Extrusion Processing of Pharmaceuticals. Handbook of Polymers for Pharmaceutical Technologies, 2015, (2):121-149
[6] 朱裕杰等. 醋酸羟丙甲纤维素琥珀酸酯在难溶性药物制剂开发中的应用进展. 药品评价, 2021,18(14): 836-839
Subscribe to be the first to get the updates!