In previous articles, we have explored prevalent techniques for producing Amorphous Solid Dispersion (ASD), such as spray drying and hot melt extrusion, highlighting their respective benefits, limitations, and practical applications. Once ASD is formulated, it is crucial to characterize it to assess the distribution of the drug within the carrier matrix. Given that ASD exists in a high-energy and unstable state, there is a propensity for drug molecules to aggregate and form nuclei, with microcrystals progressively enlarging into fully-fledged crystals, eventually culminating in a stable crystalline form. Consequently, it is imperative to diligently monitor alterations in their state and conduct thorough evaluations focusing on solubility, dissolution rate, and stability. The characterization of ASD commonly employs various analytical techniques, including X-ray powder Diffraction (XRPD), Differential Scanning Calorimetry (DSC), Polarized Light Microscopy (PLM), Scanning Electron Microscopy (SEM), Infrared Spectroscopy (IR), Raman Spectroscopy (RM), and Solid-state Nuclear Magnetic Resonance (ssNMR). These methods provide essential insights into the physical and chemical properties of ASD, facilitating an understanding of its behavior under various conditions.
X-Ray Powder Diffraction (XRPD) patterns function similarly to fingerprints, characterized by unique attributes such as the number, position, intensity, and geometric arrangement of diffraction peaks. Crystalline substances display distinct, sharp diffraction peaks indicative of their structured molecular order. Conversely, in Amorphous Solid Dispersion (ASD), the drugs are in an amorphous state, which typically manifests as broad, halo-like peaks rather than sharp, well-defined peaks. If the drug and carrier are only physically mixed rather than chemically integrated, the XRPD pattern of the mixture generally appears as a straightforward superimposition of the diffraction patterns of each individual component, reflecting their separate identities within the mixture.
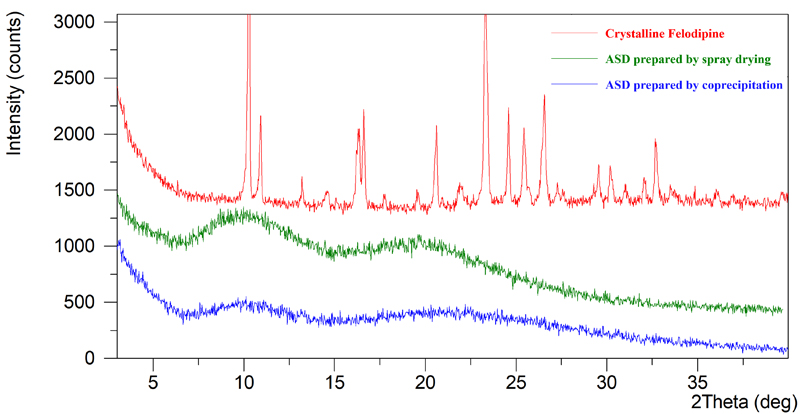
Figure 1: XRPD Comparison of Crystalline Felodipine and Felodipine ASDs Prepared by Different Methods
Thermal analysis characterizes the physical properties of a system as they evolve with temperature changes, employing Differential Scanning Calorimetry (DSC) as the predominant method. DSC quantifies the heat flow associated with a substance as it is heated or cooled under controlled conditions to keep the sample and a reference at the same temperature. The heat signals depicted in DSC curves are indicative of phenomena such as melting, recrystallization, phase transitions, and decomposition of the sample. Crystallines, for instance, typically display distinct melting signals indicative of their structured lattice. In the absence of a melting peak, the sample is likely in an amorphous form. For amorphous materials, the glass transition temperature (Tg) can be precisely determined using modulated DSC (mDSC), which provides enhanced resolution and sensitivity. Tg serves as a critical indicator of molecular mobility within ASD. Above Tg, the amorphous material is in a rubbery state, increasing the propensity for recrystallization as molecular movements become more frequent and extensive. Conversely, at temperatures significantly below Tg, the solid dispersion is more stable due to reduced molecular activity, leading to slower aging processes. Figure 2 illustrates DSC/mDSC thermograms comparing crystalline Felodipine and various ASDs of Felodipine prepared by different techniques. The crystalline API exhibits a sharp melting peak, whereas the amorphous samples show step-like Tg transitions, demonstrating the distinct thermal behaviors of different physical states.
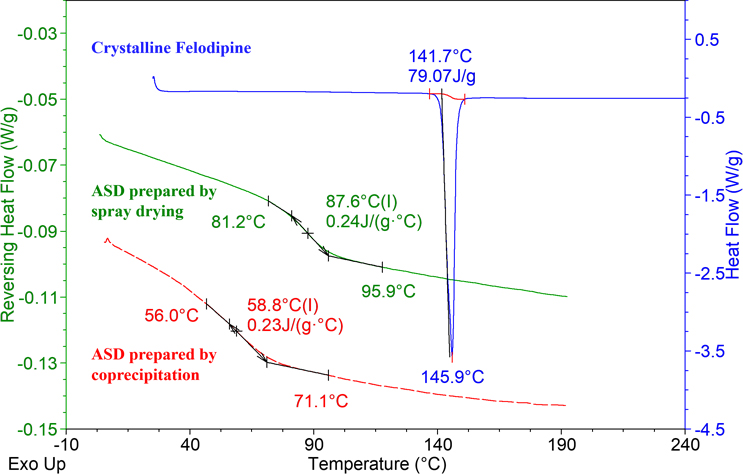
Figure 2: DSC/mDSC Comparison of Crystalline Felodipine and Felodipine ASDs Prepared by Different Methods
3.1 Polarized Light Microscopy (PLM)
Polarized Light Microscopy (PLM) is a valuable technique for visually identifying and distinguishing between crystalline and amorphous structures within samples by detecting birefringence. This method not only aids in recognizing the presence of crystallinity but also provides detailed insights into the morphology and particle size distribution of the samples. Figure 3 showcases PLM images comparing crystalline Felodipine with Felodipine Amorphous Solid Dispersions (ASDs) prepared using various techniques. Crystalline Felodipine displays pronounced birefringence, indicative of its ordered structure, whereas the amorphous samples exhibit a relative absence of light patterns, appearing darker between crossed polarizers. Furthermore, variations in particle size and morphology are apparent among the amorphous samples produced by different methods, highlighting the impact of preparation techniques on the physical properties of ASDs.

Figure 3: PLM Comparison of Crystalline Felodipine and Felodipine ASDs Prepared by Different Methods
3.2 Scanning Electron Microscopy (SEM)
Scanning Electron Microscopy (SEM) provides high-resolution imaging, offering detailed and direct insights into the morphology and surface characteristics of materials. Figure 4 presents SEM images comparing crystalline Felodipine with Felodipine Amorphous Solid Dispersions (ASDs) prepared using different carriers via spray drying. In these images, crystalline Felodipine is visible as rod-shaped particles, indicative of its structured lattice. In contrast, the ASD samples produced through spray drying display collapsed spherical particles. These particles are characterized by a smaller size and a larger surface area, attributes that significantly enhance the dissolution rate of the drug.

Figure 4: SEM Comparison of Crystalline Felodipine and Felodipine ASDs Prepared with Different Carriers via Spray Drying
4.1 Infrared Spectroscopy (IR)
Different functional groups in substances display unique infrared absorption peaks. In cases where there is no interaction between the drug and its carrier, the infrared spectrum of the Amorphous Solid Dispersion (ASD) typically mirrors that of a physical mixture. However, when an interaction occurs between the drug and the carrier, this is reflected in the infrared spectrum through shifts in absorption peaks or variations in peak intensity. These changes are valuable for substance identification, allowing for a detailed analysis of the chemical interactions within the ASD.
4.2 Raman Spectroscopy (RM)
Raman spectroscopy, a vibrational scattering technique, is particularly effective in detecting nonpolar groups where infrared absorption may be less prominent. This method can serve as a complementary tool to infrared spectroscopy, enhancing structural analysis and phase identification. By integrating Raman spectroscopy, more comprehensive and detailed results can be achieved, providing a fuller understanding of molecular interactions and material composition.
4.3 Solid-state Nuclear Magnetic Resonance (ssNMR)
Solid-state Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical tool for both qualitative identification and quantitative analysis of crystalline and amorphous forms in solid samples. This technique offers valuable structural insights by measuring dipolar interactions, spin diffusion, and relaxation times, all without the need for sample destruction. Furthermore, solid-state NMR can measure nuclear magnetic resonance relaxation times, providing critical information about molecular mobility within the sample. This capability is particularly useful for understanding the crystallization rate of amorphous drugs. Additionally, solid-state NMR is instrumental in studying drug-polymer interactions, making it a versatile and essential method in the characterization of pharmaceutical solids.
1. 吕杨, 杜冠华. 晶型药物. 北京:人民卫生出版社,2009.
2. Ron Liu. 难溶性药物制剂技术. 刘荣译. 北京:化学工业出版社,2021.
3. Tambe S, Jain D, Meruva S K, et al. Recent advances in amorphous solid dispersions: preformulation, formulation strategies, technological advancements and characterization[J]. Pharmaceutics, 2022, 14(10): 2203.
4. Anane-Adjei A B, Jacobs E, Nash S C, et al. Amorphous solid dispersions: Utilization and challenges in preclinical drug development within AstraZeneca[J]. International Journal of Pharmaceutics, 2022, 614: 121387.
5. Guo Y, Shalaev E, Smith S. Physical stability of pharmaceutical formulations: solid-state characterization of amorphous dispersions[J]. TrAC Trends in Analytical Chemistry, 2013, 49: 137-144.
Subscribe to be the first to get the updates!