Technical Articles
11 Apr 2025
Crystal’s Perspective: Polymorph Patent Landscape of 28 FDA-Approved Small Molecule Drugs in 2024
11 Apr 2025
 Solid Form Screening
Our solid form screening technology applies to polymorph screening, salt screening and cocrystal screening. Our screening technology is to design various crystallization conditions according to the physicochemical properties of the target compound and factors affecting drug crystallization, to screen and discover the most comprehensive solid form for subsequent evaluation and development. Our proprietary solid form screening technology has been applied to over 1,500 different drug molecules and proven to be efficient and effective in screening and identifying the most optimal solid form for development.
Solid Form Screening
Our solid form screening technology applies to polymorph screening, salt screening and cocrystal screening. Our screening technology is to design various crystallization conditions according to the physicochemical properties of the target compound and factors affecting drug crystallization, to screen and discover the most comprehensive solid form for subsequent evaluation and development. Our proprietary solid form screening technology has been applied to over 1,500 different drug molecules and proven to be efficient and effective in screening and identifying the most optimal solid form for development.
 Solid Form Developability Evaluation
Solid form developability evaluation technology is to evaluate the developability of crystal forms, including polymorphs, salts and cocrystals obtained in the solid form screening experiments and analyze potential risks and challenges of development. Through years of technical accumulation, Crystal Pharmatech has formed a complete evaluation system for solid form developability.
Solid Form Developability Evaluation
Solid form developability evaluation technology is to evaluate the developability of crystal forms, including polymorphs, salts and cocrystals obtained in the solid form screening experiments and analyze potential risks and challenges of development. Through years of technical accumulation, Crystal Pharmatech has formed a complete evaluation system for solid form developability.
 Absolute Structure Determination
Single crystal growth and structure determination technology are the main methods to determine the absolute configuration of drug molecules and identify the crystal forms absolutely. The single crystal culture and analysis technology firstly obtain high-quality crystals through a variety of experimental single crystal growth methods. The main single crystal growth methods include slow evaporation, gas-liquid infiltration, slow cooling and liquid surface diffusion.
Absolute Structure Determination
Single crystal growth and structure determination technology are the main methods to determine the absolute configuration of drug molecules and identify the crystal forms absolutely. The single crystal culture and analysis technology firstly obtain high-quality crystals through a variety of experimental single crystal growth methods. The main single crystal growth methods include slow evaporation, gas-liquid infiltration, slow cooling and liquid surface diffusion.
 Crystallization Process Development
On the basis of in-depth research on the properties of the crystal form itself and the transformation relationship between crystal forms, Crystal Pharmatech's crystallization process development technology realizes the selection and control of the crystallization system, crystallization conditions and post-processing conditions, so as to obtain the target crystal form at the same time taking into account the API particle properties, yield and cost, etc.
Crystallization Process Development
On the basis of in-depth research on the properties of the crystal form itself and the transformation relationship between crystal forms, Crystal Pharmatech's crystallization process development technology realizes the selection and control of the crystallization system, crystallization conditions and post-processing conditions, so as to obtain the target crystal form at the same time taking into account the API particle properties, yield and cost, etc.
 Mol2Med™ Integrated Services
Crystal Pharmatech are a specialized CRO/CDMO focused on Solid State Research, Pre-Formulation, Formulation Development and Manufacturing. Our strength is our focus and expertise in these specialties...
Mol2Med™ Integrated Services
Crystal Pharmatech are a specialized CRO/CDMO focused on Solid State Research, Pre-Formulation, Formulation Development and Manufacturing. Our strength is our focus and expertise in these specialties...
 AAPS 2024 PHarmSCi 360
Meet with industry leadership to discuss:Developability AssessmentAPI Form and Solid-State ResearchFormulation DevelopmentClinical and Commercial ManufacturingBiologics Analytical ServicesDate: Octobe...
AAPS 2024 PHarmSCi 360
Meet with industry leadership to discuss:Developability AssessmentAPI Form and Solid-State ResearchFormulation DevelopmentClinical and Commercial ManufacturingBiologics Analytical ServicesDate: Octobe...
 Concerned about mRNA -LNP Integrity during International Shipping?
Several batches of mRNA-LNP samples, shipped by CATUG (CDMO in China), were subjected to Quality Control-style testing performed at the receiving site (Crystal Bio, New Jersey, USA) to address the con...
Concerned about mRNA -LNP Integrity during International Shipping?
Several batches of mRNA-LNP samples, shipped by CATUG (CDMO in China), were subjected to Quality Control-style testing performed at the receiving site (Crystal Bio, New Jersey, USA) to address the con...
 Improving the Manufacture of mRNA Biologics
Improving the Manufacture of mRNA Biologics
 Home
Crystal Pharmatech's Mol2Med™ First-Time-Right approach guarantees a robust API form and a scalable manufacturing process, culminating in a First-Time-Right formulation for Phase I. Streamlining the transition to future clinical studies upon Phase I success, this innovative approach sets the foundation for optimized drug development and success beyond.
Home
Crystal Pharmatech's Mol2Med™ First-Time-Right approach guarantees a robust API form and a scalable manufacturing process, culminating in a First-Time-Right formulation for Phase I. Streamlining the transition to future clinical studies upon Phase I success, this innovative approach sets the foundation for optimized drug development and success beyond.
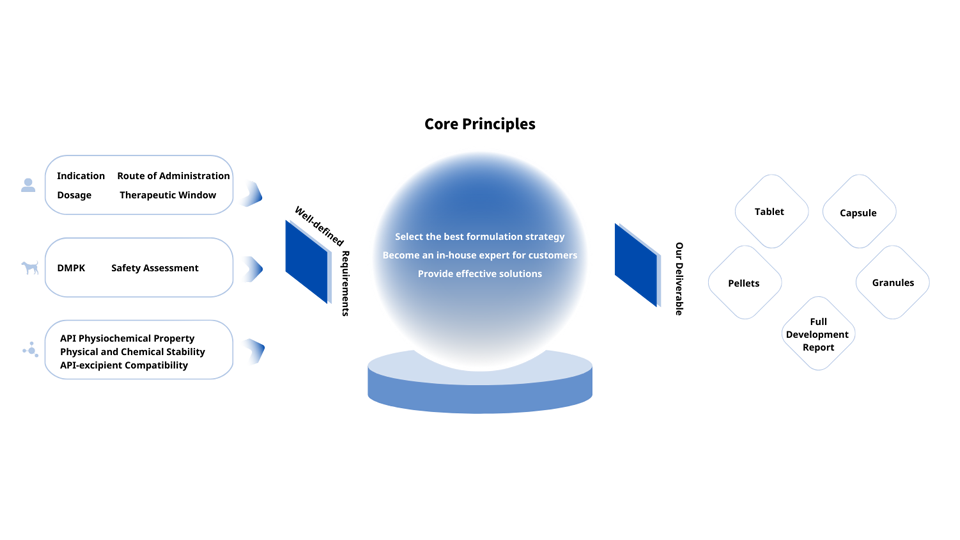 Formulation Development
We guide our customers into making smarter formulation choices. Ultimately, this reduces the risk of clinical failure and ensures your product reaches the clinic rapidly via the most efficient route.
Formulation Development
We guide our customers into making smarter formulation choices. Ultimately, this reduces the risk of clinical failure and ensures your product reaches the clinic rapidly via the most efficient route.
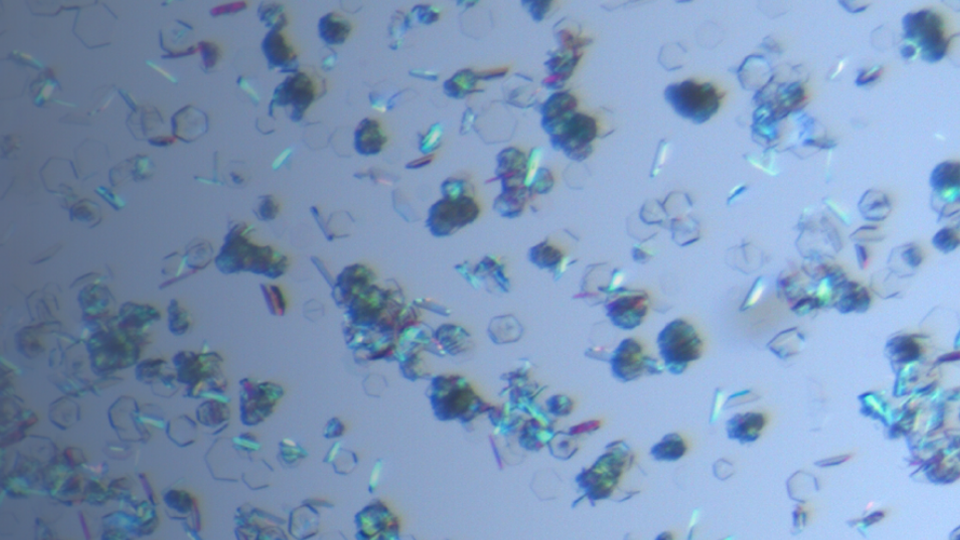 Solid Form Screening and Selection
Solid form is a general term that refers to both crystalline and amorphous materials. The solid form will impact active pharmaceutical ingredient (API) development properties such as solubility, dissolution rate, stability, hygroscopicity, and bioavailability.
Solid Form Screening and Selection
Solid form is a general term that refers to both crystalline and amorphous materials. The solid form will impact active pharmaceutical ingredient (API) development properties such as solubility, dissolution rate, stability, hygroscopicity, and bioavailability.


