11 Apr 2025
DPC 961 (Fig. 6) was a development compound indicated for the treatment of HIV infections and was developed as a neutral molecule (Staszewski et al. 1999). The compound was designated as Biopharmaceutics Classification System (BCS) II, with high permeability, low aqueous solubility and therefore would display dissolution limited behavior (Aungst et al. 2002). As a result, physicochemical characteristics such as particle size, crystal form, and surface area may have had a direct impact on bio-performance. Early in development, this compound had been known to exist in many solvated forms in addition to a single, anhydrous crystal form (Form I) (Desikan et al. 2005). Preliminary screening work had never identified crystallization solvents that directly isolated Form I. All pathways to Form I involved forming a solvate and then de-solvating to obtain Form I. The first 29 development batches of DPC 961 involved isolation of the API through crystallization from toluene/heptane, followed by re-crystallization from methanol (MeOH). Anhydrous Form I was the product in all 29 batches; however, this form was not directly crystallized, but instead, formed through de-solvation of the stoichiometric MeOH solvate by elevated temperature drying. On the 30thbatch, a lower melting crystal form, anhydrous Form III, was the product. Form III was determined to be enantiotropically related to Form I, with Form III being the low temperature stable polymorph with transition temperature between 120 and 174 °C, as determined from DSC data using Burger’s rules. A van’t Hoff investigation had not been performed in this polymorph system, presumably since no solvent had been found that had not formed a solvate with either Form I or III.
Fig. 6: Structure of DPC 961
After the serendipitous discovery of Form III, Form I was never again manufactured at large scale. When the desolvation employed in the first 29 batches was attempted after Form III was discovered, the product was now Form III, and not Form I. Form I had been prepared on small scale by heating Form III above melting point, but a manufacturing process could not be developed. This circumstance was a clear example of the phenomenon labeled as a “disappearing polymorph” (Dunitz and Bernstein 1995). Due to this change in form, researchers were now left with Form III. Since the compound was BCS II, dissolution may have critically impacted bioperformance. Thus, the first set of experiments necessary when Form III was discovered and realized to be the future chosen phase was to understand bio-relevant dissolution and solubility. Fortunately, Form III had comparable aqueous solubility and intrinsic dissolution rates. An oral absorption study in animals was necessary to confirm that bio-performance would not be impacted by the crystal form change. When Form I and Form III were formulated into tablets and orally administered to dogs at 100mpk, the oral absorption profiles were statistically identical (Fig. 7). If this had not been the case, and formulated Form I resulted in a unique absorption profile compared to Form III, a human bridging study would have been necessary. The cost and program delays would have been substantial. While statistics are not available, the chances of Form III and Form I having identical bio-performance for a BCS II compound was likely to be low. The more probable result would have been distinct solubility and/or dissolution differences between the polymorphs. Even though a clinical bridging study was not necessary after the polymorph change, the research team still had to develop a unique API isolation process and update analytical methods, in addition to providing the necessary data to prove polymorph stability and bio-equivalence, which would have likely taken a minimum of six months to perform.
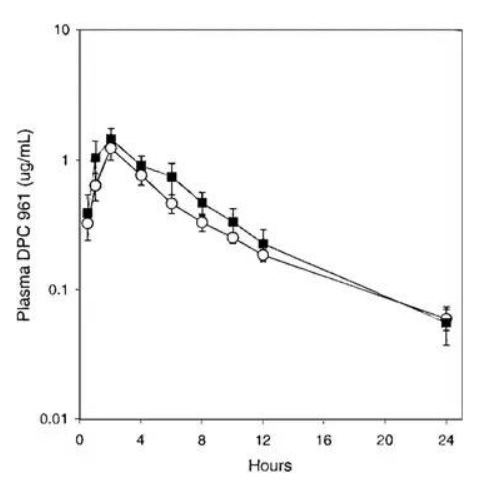
Fig. 7: Comparison of DPC 961 oral absorption in dogs administered 100 mg tablet formulations prepared with polymorph form I (○) or form III (■). (used with permission from Ref (Aungst et al. 2002))
The lessons learned from this case study would vary based on the company’s risk-management strategy. It has been generally accepted that isolating the final crystal form through desolvation would be a non-ideal process; rather, a process where the final form has been directly nucleated and grown (with or without seeds) would be preferred. However, there have been compounds that, when developed initially, have only appeared to form solvates. These solvates may or may not have had the potential to de-solvate to a physically stable anhydrous crystal form. In this case study, Form I appeared to exhibit adequate physical stability, but was not found to be an anhydrous crystal form that could have been directly nucleated and grown in an appropriate solvent system. Due to the speed of the program, it could have easily been argued that the process was robust in isolating Form I, as 29 batches had been completed without incident. The opportune discovery of Form III may have occurred due to a variety of causes including, but not limited to: impurity differences (either level or actual type of impurities) in the process stream, unique levels of supersaturation, or foreign particle providing heteronuclear templates for nucleation. It is not known whether extensive screening early in the program would have uncovered Form III. However, many compounds that have only been isolated as a solvate had often times masked an anhydrous crystal form that had been anticipating the right trigger in order to be discovered. Therefore, an appropriate and diligent level of crystal form screening should be applied to this type of compound, especially since the compound was designated at BCS II. The screening strategy should have involved conditions attempting to avoid solvate formation (Campeta et al. 2010). This “fast-track” compound could be deemed a “bad” scenario when the time delays and increased costs to the project have been added to the development plan.


