22 Jan 2013
Hot melt extrusion (HME) is a common formulation technology for amorphous solid dispersions (ASD) with advantages such as continuity, high production efficiency, low energy consumption, and environmental friendliness. It can meet the formulation requirements of various poorly soluble drugs. Hot melt extrusion utilizes a screw extruder to fully mix the API and polymer carrier in a molten state under the action of heat and mechanical stress, and extrude and cool down to form an amorphous solid dispersion.
Hot melt extrusion is a one-step manufacturing process that does not require solvents, but introduces mechanical stress, especially high shear forces. The process combines principles of mechanics, thermodynamics, rheology, and materials science. Due to the viscoelasticity of polymer materials and the thermal stability of the API and carrier, the design and control of the extrusion process are also very complex, including feed design, screw specifications and combinations, residence time distribution (RTD), and types of exit molds. In addition, downstream processes such as cooling, grinding, and tablet coating have a significant impact on product stability and bioavailability.
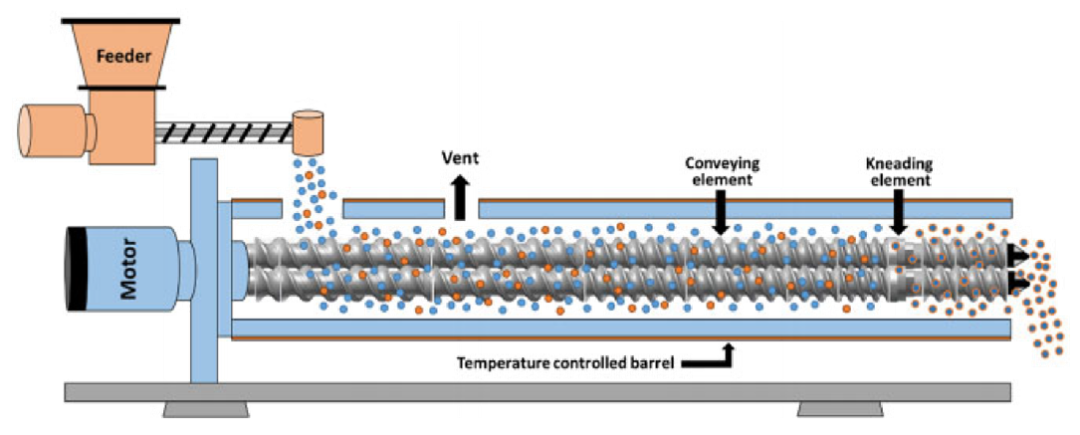 Hot melt extrusion process
Hot melt extrusion process
Advantages
Hot melt extrusion can produce solid formulations with uniform shape and size.
The drug release of hot melt extrusion formulations is uniform, leading to high bioavailability.
Hot melt extrusion can be applied to drugs with good thermal stability.
Continuous production process with high process repeatability, eliminating batch-to-batch variations.
PAT technology can be used for online monitoring.
Can be applied to large-scale industrial production.
No solvent consumption, environmentally friendly.
Disadvantages
Hot melt extrusion requires high temperature, which may cause decomposition and degradation of certain drugs.
Requires at least kg-level API, which is a challenge for early-stage development.
Market oral formulations utilizing hot melt extrusion technology
Olaparib
Olaparib Compound Structure
Olaparib is the first FDA-approved oral poly (ADP-ribose) polymerase (PARP) inhibitor drug, which targets the DNA damage repair pathway and uses "synthetic lethality" to kill cancer cells without affecting healthy cells. It has been approved for multiple indications such as ovarian cancer, breast cancer, prostate cancer, and pancreatic cancer. With its excellent therapeutic effects, the drug has been marketed in over 90 countries worldwide, with global sales reaching $3.7 billion in 2022.
Although the drug has become a blockbuster in the field of oncology, during the development process, due to its low bioavailability, the original research attempted various dosage forms and formulation strategies before achieving successful commercialization.
Olaparib is a BCS class IV drug and a weakly acidic compound. It is basically neutral within the physiological pH range and has a low solubility in aqueous buffers (approximately 0.1 mg/mL over the pH range of 1-9). In simulated gastric and intestinal fluids, the solubility ranges from 0.12 to 0.20 mg/mL, with the highest solubility observed in postprandial simulated intestinal fluid at 0.20 mg/mL. The low solubility and permeability of the drug limit its bioavailability and clinical use.
To address the issues mentioned above, during the early development phase, the research team studied tablet and capsule formulations for the semi-solid glycerol monolaurate (LMG). In preclinical studies with dogs, it was found that the capsule formulation had better relative bioavailability. Initially, the formulation development focused on two methods to enhance the bioavailability of this poorly soluble compound: liquid-filled capsules and crystalline solid dispersion capsules. Due to the inability to find suitable solvent systems and considering potential issues such as capsule leakage, stability, and interactions between excipients and the active substance in liquid-filled capsules, the decision was made to develop crystalline solid dispersion capsules.
To increase the drug's exposed surface area, the research team adopted a micronization strategy for the active pharmaceutical ingredient (API). Despite the formulation optimization, the percentage of the active substance remained low.
Considering the capsule dosage form, patients needed to take eight capsules each time (50 mg specification, twice a day, totaling 400 mg each time). This dosing regimen was not ideal for patients. To reduce the burden on patients, the research team adjusted the drug dosage form again. Initially, they attempted to develop standard immediate-release tablets using micronized olaparib. However, even with micronized olaparib, the dissolution rate of this type of dosage form remained limited.
Since olaparib has low solubility in aqueous media (≤0.1 mg/mL), the development direction for the dosage form was to improve solubility. Subsequently, through systematic studies of several different enhanced formulations, it was found that the amorphous solid dispersion of olaparib prepared by hot-melt extrusion (HME) significantly improved bioavailability compared to other formulations (i.e., using glycerol monolaurate (LMG) capsule formulations). The amorphous solid dispersion formed by the active ingredient and the polymer carrier not only increased the loading of the active substance in a single dosage unit, reducing the number of doses (changed to twice a day, 300 mg each time, i.e., 2 tablets), but also significantly improved the drug's solubility and bioavailability. This resulted in a lower daily dose of the active ingredient in tablet form compared to the capsule formulation, with the 300 mg tablet achieving exposure levels equivalent to the original marketed capsule (which contained a significant amount of surfactant). This significantly improved patient compliance and marked a product upgrade.
Crystal's Pharmaceutical CDMO division boasts a core team with backgrounds in polymer materials, chemical engineering, and mechanical engineering. Guided by the principles of rheology and engineering, our team has extensive expertise in the field of HME. We have previously developed HME-based formulation technology platforms, including immediate-release and sustained-release formulations, with a wealth of experience in process scale-up and commercial production. We have world-class equipment and analytical instruments for rheological research, formulation screening, and process development, from small-scale trials to commercial production. We sincerely hope to assist innovative pharmaceutical clients in overcoming the barriers posed by poorly soluble drugs using the hot-melt extrusion method.