Origin of Chocolate History
For some reason, chocolate seems to be inherently regarded as a symbol of romance, appearing in literary works and love connections between people. Its unique rich and silky, sweet and slightly bitter flavor, resembles the sweetness and bitterness of love, making people unable to stop and bringing joy to the heart. Ancient chocolate was still a symbol of privilege and status, a royal drink of the Indian kingdom, with a unique name "Chocolate", which means hot drink. It was not until 1849 when the Fry father and son chocolate company produced the first delicious chocolate bar, that chocolate became an affordable solid product. With the popularity of cocoa bean planting and the mechanization of grinding processes, it shed its glamorous façade and became a star product with a wider audience.


The protagonist behind the delicious and silky chocolate
The combination of sweetness and bitterness in chocolate brings a delicate and smooth taste to the palate, making it difficult for people to resist. When you enjoy it endlessly, have you ever considered the reason why? The mystery lies in one of the main ingredients of chocolate - cocoa butter. Cocoa butter itself is bitter (think about how bitter the dark chocolate with high cocoa butter content you usually eat is), but various substances, such as sugar, milk, and flavorings, are added during the refining process, producing a sweet taste. In addition, cocoa butter has the "magical" property that gives chocolate its melting characteristic of "not melting in the hand, but melting in the mouth."
Let me explain in detail.
The chemical composition of cocoa butter
The chemical composition of cocoa butter primarily contains monounsaturated triglycerides (TAGs), which are composed of two saturated fatty acids (palmitic acid and stearic acid) and one unsaturated fatty acid (oleic acid) and glycerol. This results in a relatively low fat content that has simple structure confirmation. It melts quickly in a small temperature range and is roughly within the range of room temperature to an oral temperature. Cocoa butter also contains 1%~2% triglycerides with three saturated fatty acids (whose saturated fatty acid is mainly palmitic acid or stearic acid), which require a higher melting temperature, and about 5%~20% triglycerides with two unsaturated fatty acids (containing two oleic acid molecules), which have a lower melting temperature and are often liquid at room temperature. The combination of these three types of TAGs creates the unique melting characteristic of cocoa butter.
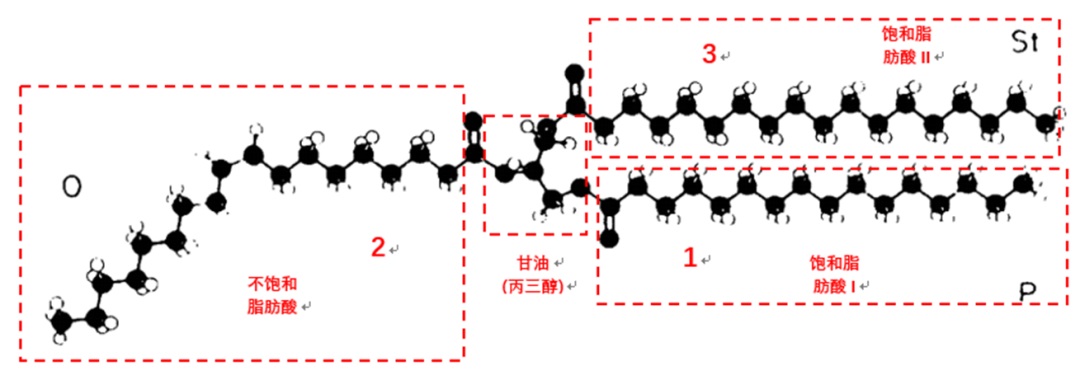
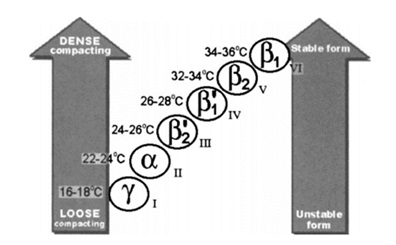
The phenomenon of cocoa butter's polymorphism
Cocoa butter has various crystal forms, as identified by the crystallization process development. From a crystallographic perspective, cocoa butter exists in multiple crystal forms, which are divided into Form I, II, III, IV, V, VI or γ, α, β2', β1', β2, β1 (the former is the naming rule proposed by Wille's and Lutton's teams, while the latter is proposed by Larsson's team) in order of melting point (and stability) from low to high. Types I to IV have low melting points and are unstable, and will transform into stable types under certain temperatures and time; while types V and VI are hard and brittle, with a melting temperature closer to body temperature, which is the reason why chocolate does not melt in the hand but melts in the mouth. However, the most stable VI-type crystal has a large particle size, resulting in a rough texture and a waxy taste. At the same time, the surface of this type of chocolate will have a layer of white frost, which looks like it has deteriorated, and is not recommended. Therefore, the metastable V type stands out as the protagonist of the chocolate configuration. This was first verified in 1966 by Wille's and Lutton's teams, who identified the good-tasting chocolate crystals as all V type.
Subscribe to be the first to get the updates!