How can physiologically-based pharmacokinetic (PBPK) modeling maximize ADME performance and identify a path forward for GLP Tox and FIH formulations?
GastroPlus incorporates in-vivo and in-vitro data to build animal models. The animal models can be scaled to human models, providing advanced insights on ADME performance in animals and humans.
Case studies on insights that drive more efficient development…
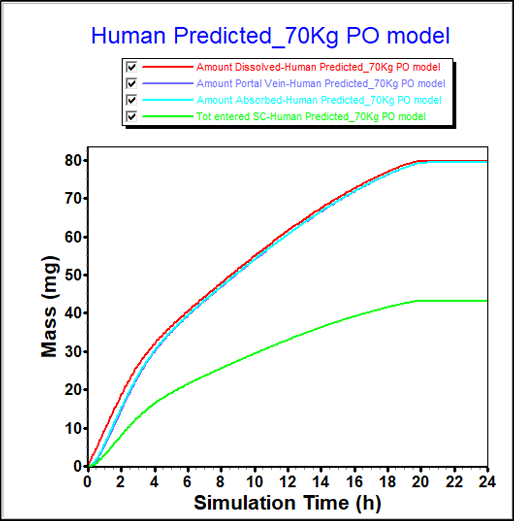
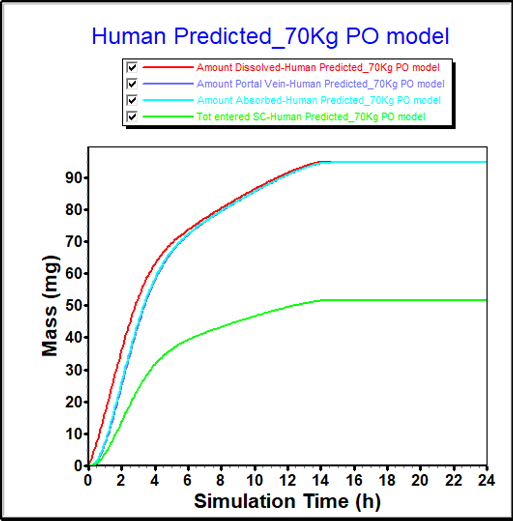
From in-vitro data, FaSSIF and FeSSIF solubilities can indicate a food effect. However, the impact of that food effect is difficult to quantify from solubility measurements alone.
PBPK modeling provides a more detailed understanding of the food effect for this compound, showing a significant increase in bioavailability.
These insights can guide study design and direct formulators to pursue maximizing this effect in their formulation development.
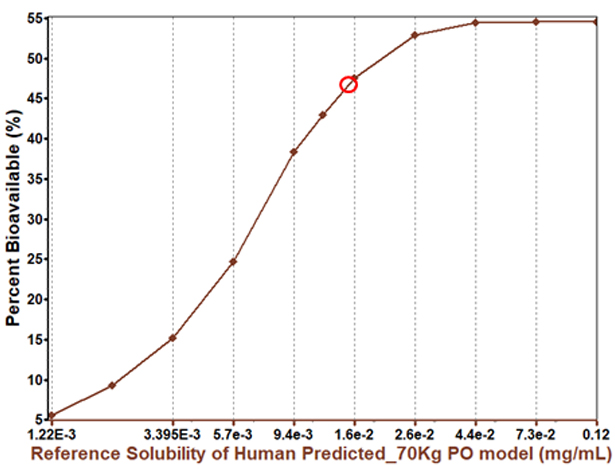
There are many options to consider when looking to increase a compound’s solubility. Solubility enhancement can yield significant improvements in bioavailability, but it is not always necessary.
In this case, increasing the compound’s solubility will increase bioavailability, but with a diminishing return. This indicates that bioavailability may not be entirely solubility limited.
The PBPK insights for this parameter help drug innovators and formulators understand when it is worthwhile to pursue solubility enhancement and when to consider other factors affecting bioavailability.
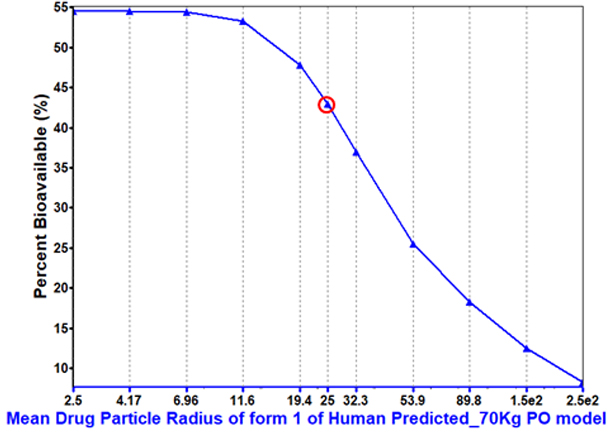
Particle size can play a key role in increasing bioavailability, particularly for dissolution-rate limited compounds.
This compound sees significant benefit from maximizing the dissolution-rate with decreased particle size.
Understanding the significance of this parameter helps inform drug developers whether particle size reduction is necessary and the optimal range for controlling particle size.
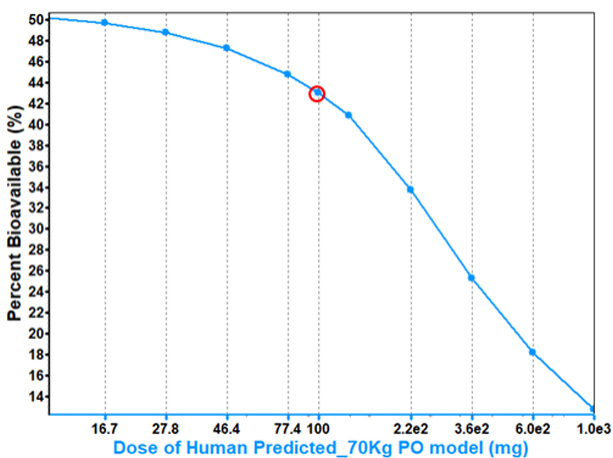
If we want to increase bioavailability, can we simply increase the dose?
In some cases, a higher dose will result in higher bioavailability.
However, in other cases, bioavailability can decrease with increasing dose for a solubility limited compound due to its higher potential for precipitation.
Understanding the dose at which precipitation is a risk can guide study design and direct formulators on the necessity for measures preventing precipitation.
GastroPlus parameter sensitivity offers a more efficient alternative to trial-and-error PK studies as drug innovators look to rapidly determine the effects of various parameters on bioavailability.
These early insights, combined with experienced solid-state chemists and formulators, can provide invaluable insights for lead candidate selection and the optimal GLP Tox and FIH formulation approaches.
Crystal Pharmatech has in-house experts in GastroPlus PBPK modeling and regularly helps innovators incorporate PBPK modeling into their programs for more efficient and effective development.
If you would like to learn more about how this powerful tool can benefit your program, please reach out to BD_Global@CrystalPharmatech.com.
Subscribe to be the first to get the updates!