Development of Frozen Food
It is hard to tell when various brands of frozen dumplings, glutinous rice balls, hand-grabbed pancakes, and frozen meats have filled up the freezer compartment of our refrigerators. Frozen foods are ubiquitous in supermarkets, hot pot restaurants, and online food delivery platforms. After work, compared to spending two hours shopping, washing, cooking, and cleaning dishes, I prefer a bowl of delicious dumplings that can be ready in just 15 minutes. Frozen foods have indeed freed up our hands and brought much convenience to our lives. In addition, due to the repeated outbreaks of the pandemic nowadays, it has become a trend to stock up on food and meat in the freezer for peace of mind. According to data provided by the National Bureau of Statistics, in 2019, the operating income of frozen food industrial enterprises above a certain scale in China reached 2.01 trillion yuan, a year-on-year increase of 9.5%, which was 5.7 and 5.3 percentage points higher than the increase rates of all industrial enterprises above a certain scale and the national food industry operating income respectively, achieving a high-speed growth. According to the prediction of China Market Research Puhua Institute, the frozen food industry in China will still be in the growth stage in the next ten years, with an expected compound annual growth rate of about 10%.



The preservation of frozen food itself is an effective, long-lasting, and cost-effective preservation method. But does this method have any impact on the quality of food? One day, as the author was in a hurry to cook dinner, he used hot water to thaw frozen pork and saw half a bag of blood water and some rotten meat fibers, which led to contemplation. The fried pork tasted bland. Upon further reflection, the beef repeatedly frozen in the refrigerator had long lost its original blood-red color. It is believed that everyone has had similar experiences, which are easily overlooked. Based on this, the author fully utilized his scientific talent and investigated the culprit behind all this - ice crystals. Now, let's hear its "defense"!
Ice crystal: Hello everyone, I am Ice Crystal. I apologize for the impact I have had on the quality of your food. Please allow me to explain the following from several aspects.
The Origin: The Mechanism and Process of Ice Crystals Formation from Water


It is common knowledge that the mother of ice crystals is ice, and ice comes from the freezing of water. But do you know the principle behind it? The key step in the formation of ice crystals is nucleation.
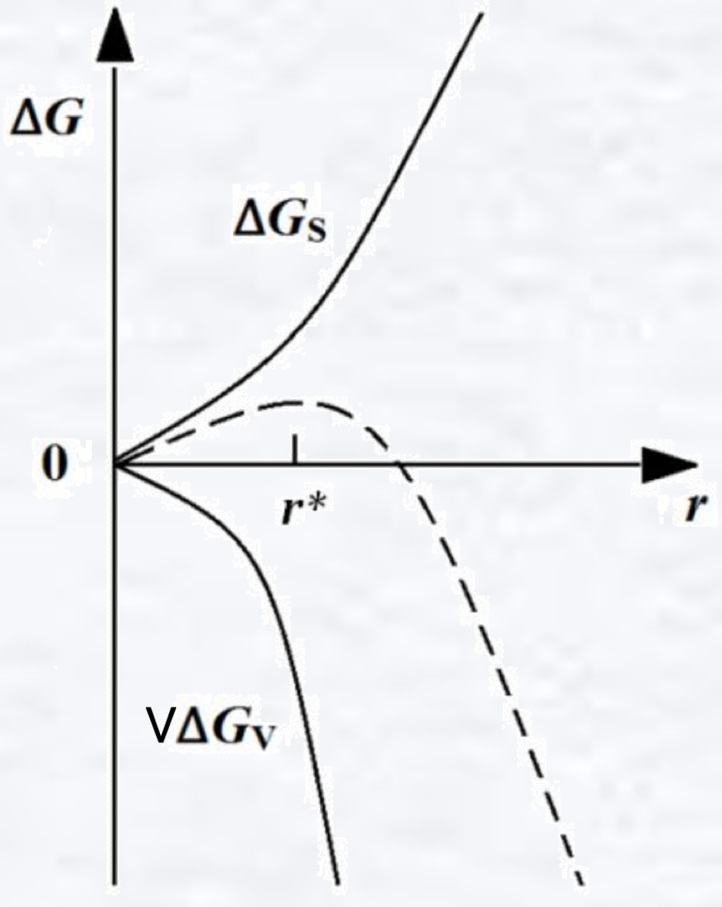
According to the "classical nucleation theory", the crystallization of pure supercooled water is a homogeneous nucleation process, that is, it relies on its own atomic motion to form a nucleus. If a fine crystal of radius r is formed in supercooled water, the energy in this region will change, consisting of two parts: the volume free energy decreases due to the transformation of a certain volume of liquid into a solid; the surface of the fine crystal forms a liquid-solid phase interface, which increases the surface free energy. These two components have opposite impacts on the total free energy, which needs structure confirmation.
ΔG = VΔGv + ΔG
= 4πr3·ΔGv/3 + 4πr2σ
ΔGv is the difference between the solid-liquid Gibbs free energy per unit volume, which is negative in supercooled water; σ is the interfacial energy, which is constant and positive. When fine crystals appear, their ability to grow depends on whether the free energy of the system decreases when their volume increases. When r is small, the surface free energy dominates, and as r increases, the system Gibbs free energy rises, and this spontaneous process decreases until it disappears and the crystal dissolves; when r is large, the bulk free energy dominates, and as r increases, the system Gibbs free energy decreases, and this fine crystal can grow and is called a nucleus.
Thus there exists a critical nucleation radius r*, which makes a point of great value for ΔG, i.e., satisfying dΔG/dr = 0, yielding r* = -2σ/ΔGv.
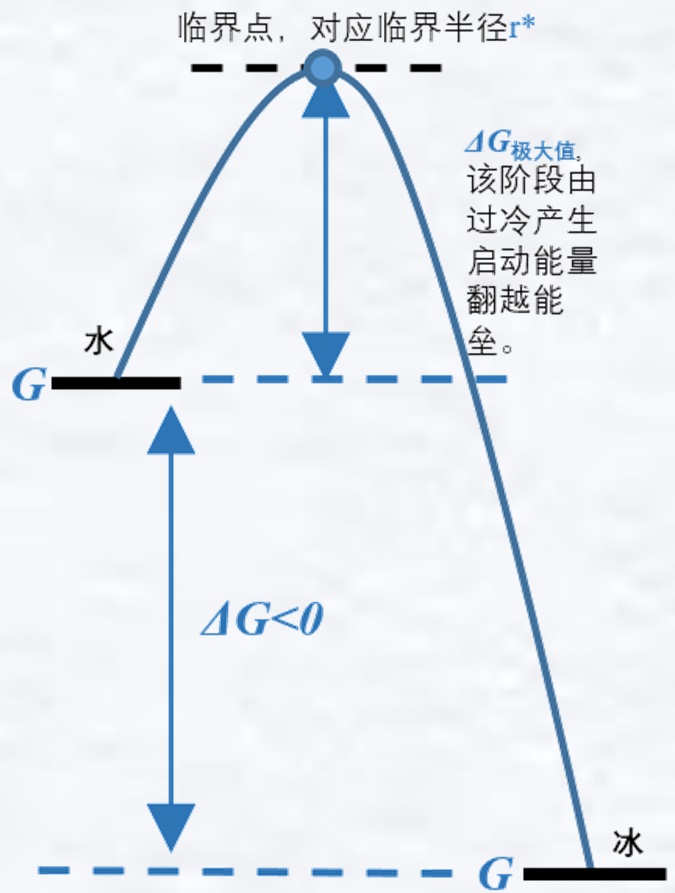
Text above: critical point, corresponding to the critical radius r*
ΔG maxima, the stage where the start-up energy generated by subcooling overturns the energy barrier.
The extreme value of ΔG is the free energy barrier for the homogeneous crystallization of supercooled water. The energy barrier is temperature dependent, and for pure supercooled water, the free energy barrier is easily crossed below -40 °C to form homogeneous crystallization. In practice, however, water freezing is a heterogeneous nucleation process, and nucleation occurs at the contact site between the liquid and solid boundaries. The solid-liquid interface can reduce the free energy required for nucleation, and the degree of supercooling is relatively low (a temperature difference that needs to be reached for crystallization to nucleate, and the temperature has to be lowered below the freezing point), thus making it easier to crystallize.
Once nucleation has taken place, the ice crystals have to start growing. It is well known that all molecules are in constant motion, except that the molecular thermal motion of water molecules is diminished when ice is formed. However, the initial formation of the nucleus is not stable and easily dispersed by the thermal motion of other water molecules. Only when the temperature continues to drop to a certain level, the thermal motion of water molecules is further weakened to form a stable nucleus, which lays the foundation for the growth of ice crystals. The water molecules move and bind to the stable nuclei in an orderly manner, which also allows the ice crystals to grow larger and larger.
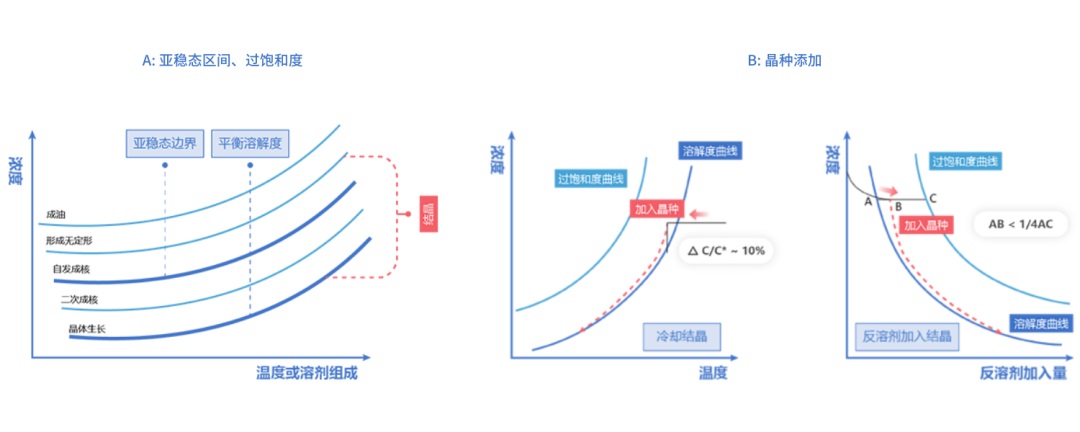 Text above: A: Sub-stable interval, supersaturation, sub-stable boundary, equilibrium solubility, concentration, temperature or solvent composition, oil formation, amorphous formation, spontaneous nucleation, secondary nucleation, crystal growth, crystallization.
Text above: A: Sub-stable interval, supersaturation, sub-stable boundary, equilibrium solubility, concentration, temperature or solvent composition, oil formation, amorphous formation, spontaneous nucleation, secondary nucleation, crystal growth, crystallization.
B: crystal species addition, concentration, solubility curve, supersaturation curve, crystal species addition, cooling crystallization, temperature, antisolvent addition to crystallization, amount of antisolvent addition.
The nucleation and growth mentioned here is also the key to control the crystallization development process. The control of the crystallization process can be started from the point of view of regulating the nucleation or growth of crystals, but usually both factors need to be controlled simultaneously. From an overall results perspective, each unit parameter (e.g., temperature reduction rate, crystalline seed addition, antisolvent addition, etc.) needs to be evaluated to determine the most critical process parameters and thus which one of nucleation or growth plays a dominant role in the crystallization process.
For the control of the crystallization process, the conventional view focuses mostly on nucleation, since the type, number and size of the initially formed nuclei are critical. However, there are multiple factors that can affect nucleation, nucleation rate and number of nuclei before growth dominates, often making the nucleation process difficult to control. Therefore, the focus of the crystallization process has to shift from nucleation control to growth control based on the control requirements of the physical properties of the final material, where the most critical control factors are supersaturation and crystal species.
Subscribe to be the first to get the updates!