04 Mar 2013
Our sincere suggestion—how to improve the quality of food affected by ice crystals
Choose frozen food
It is internationally recognized that when the central temperature of frozen food reaches -18℃ rapidly and is stably refrigerated at this low temperature, the original color, flavor and quality of the food can be maximally preserved.

There are definitely many people who hold the bias that frozen meat is not as good as fresh meat. There are always people who insist on getting up early to select the freshest meat from the market and then store it in the fridge after they can’t finish it. Did you ever think that this fresh meat, from the slaughter in the early morning until it reaches your hands at 7 or 8 o'clock, has been exposed to the environment for a long time, and a large number of microorganisms have multiplied. Moreover, the leftover fresh meat has to be put in the fridge, which has a freezing effect that cannot match professional equipment, causing further quality degradation. In this case, why not choose frozen meat from a reliable source from the beginning and thaw as much as you need to eat? Now the development of frozen equipment in China is already very mature, and the production of frozen food has increased substantially. In the near future, with further improvement of freezing/refrigeration technology, the gap between frozen food and fresh food will be negligible.
How to thaw
From the perspective of thawing, it can also alleviate the damage caused by ice crystals to some extent. The most common methods, also used in daily life, include natural thawing, water thawing (cold water/hot water), and microwave thawing. Researchers have conducted experiments investigating the effects of these thawing methods on the status and nutritional value of frozen vegetables, meat, and other foods. These studies reveal that each method has its own set of advantages and disadvantages, making it crucial to do structure confirmation and choose an appropriate method to achieve optimal results. Here are various methods that have practical significance, providing a helpful reference for practical use.
Air thawing

Using air as the thawing medium, also known as natural thawing. This thawing method has low cost and easy operation, and is suitable for large-volume meat; however, the thawing rate is slow, the meat surface is prone to discoloration, there is a certain degree of juice loss, and it will also be contaminated by dust and breed a large number of microorganisms. It is recommended to put the food to be thawed in the lower temperature in a cold storage room the day before (cold storage thawing), which can reduce the loss of juice and inhibit the growth of microorganisms. This slow thawing method is very suitable for frozen meat, mantou, bread, etc. If the situation is urgent, the frozen meat can also be placed in two-layer aluminum trays for thawing, which enhances heat transfer and achieves rapid thawing.
Water thawing

With water as the defrosting medium, the defrosting rate is faster than air defrosting. But remember not to use hot water to defrost ingredients! In this case, the food loses a lot of juice and the quality is greatly reduced. So water thawing must be used in cold water, and pork and other meat products should not be immersed directly in water as far as possible, it is best to bring the packaging to reduce microbial reproduction. However, for frozen seafood such as shrimp, eel, and tuna, soaking in still water is insufficient. Recent research shows that using this method leads to a more uniform crystallization development, lower juice loss, and smaller impact on muscle fiber tissue, optimizing the retention of frozen fish and shrimp's freshness and nutrition. Additionally, adding a little salt to the water decreases the water's freezing point, resulting in a faster thawing time without affecting the meat's freshness.
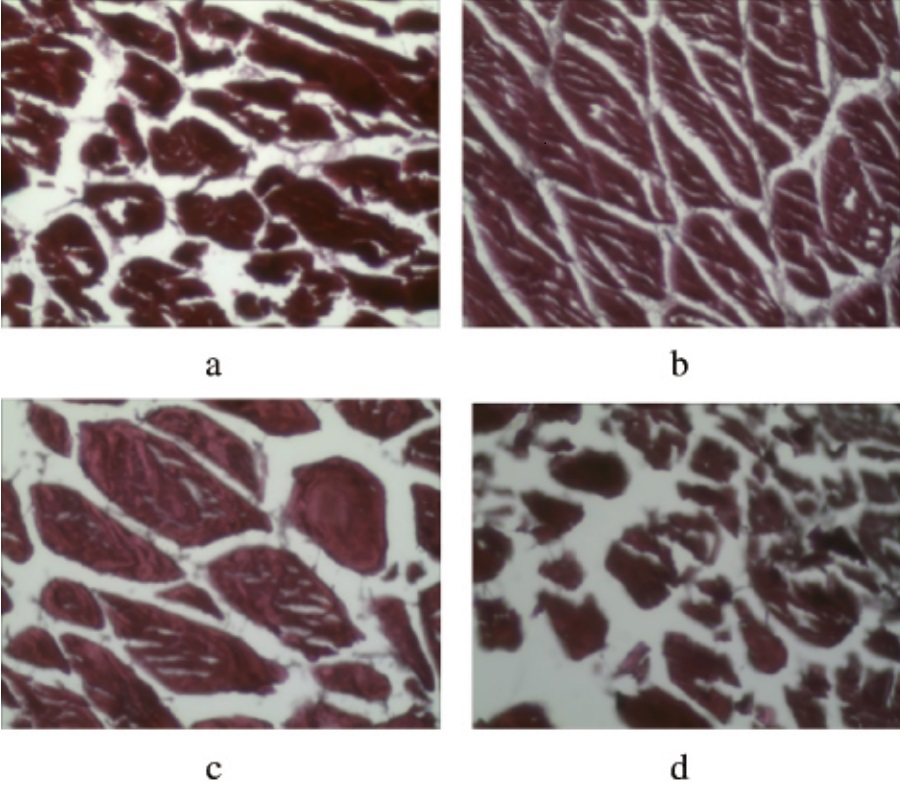
(eg. The effect of different thawing methods on tuna tissue structure: a: air thawing, fish tissue is exposed to air for a longer time, leading to the proliferation of microorganisms, decomposing tissue proteins, thus losing its original denseness and presenting a larger gap; b: hydrostatic thawing, the best effect; c: running water thawing, the ice crystals in direct contact with the water flow melt first, thawing faster, the melted liquid phase water to (d: microwave thawing, tuna muscle fiber gap is the largest, connective tissue destruction is more serious, this is because the microwave heating directly to the fish, the highest temperature, so that the protein is destroyed to a greater extent.)
Microwave defrosting

The microwave acts directly on the inside of the food, causing the molecules inside the food to collide with each other and thaw by frictional heat generation, and the surface of the food does not come into contact with the electrode, thus avoiding microbial contamination. However, there are still controversial studies on its effect on the quality of frozen food, which is commonly associated with problems such as external heat and internal cold, and uneven thawing. In the use of this method, be sure to pay attention to the microwave frequency (not more than about 700 W low-power gear, now commercially available microwave ovens will also set a special defrosting gear), thawed objects are not too thick, the surface covered with plastic wrap, immediately take immediately thawed immediately cooking. Fish and shrimp try not to use microwave defrosting, frozen fruit is still quite suitable. A large number of studies have shown that frozen strawberries, mangoes, corn, etc. put into a low-power microwave oven for a few minutes can be thawed quickly, and can maintain a greater degree of quality.
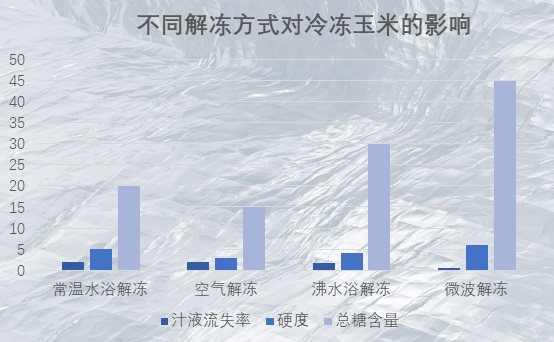
(eg. It was found that microwave thawing of corn took the least time, lost the least juice, retained the best structure, and had the highest total sugar content.)
Text above: Effect of different thawing methods on frozen corn, thawing in room temperature water bath, thawing in air, thawing in boiling water bath, thawing in microwave, juice loss rate, hardness, total sugar content.
Ultrasonic thawing

Without having to think about money, perhaps a small ultrasound machine can be acquired at home? Because in the current research, ultrasonic thawing is considered one of the best reputation among the commonly used thawing methods. Ultrasonic action on water produces thermal effect, mechanical effect and cavitation effect, on the one hand, the cavitation effect assists the thermal effect to make the heat more smoothly conducted to the food interior, on the other hand, the mechanical effect can effectively destroy the ice crystals within the food, thus accelerating the thawing to achieve the purpose of fast and stable thawing of food.
Conclusion
Of course there are more high-tech defrosting methods, as well as different defrosting methods in conjunction with the method, here is not one by one, after all, our purpose is also to be able to quickly eat a plate of thawed food taste no different from the fresh dish, too much tedious steps some of the cart before the horse, and the above knowledge is enough for daily life. So, go look at the frozen dishes in the refrigerator again, your defrosting ideas clear?