01 Jan 2018
Hot-melt extrusion (HME) is recognized as an optimal and efficient technique for manufacturing amorphous solid dispersions, characterized by its continuous processing, increased production efficiency, reduced energy consumption, and environmental sustainability. These attributes make it particularly suitable for the preparation of formulations for drugs with poor solubility. In HME, a screw extruder combines the active pharmaceutical ingredient (API) with a polymer carrier under heat, creating a molten mixture. This mixture is then extruded and cools down to form an amorphous solid dispersion.
HME, a solvent-free and single-step method, applies significant mechanical stress, especially in the form of high shear forces. The technique involves concepts from various disciplines including mechanics, thermodynamics, rheology, and materials science. The complexity of designing and controlling the extrusion process arises from the viscoelastic properties of polymers. Key factors like the feed design, screw specifications and configuration, residence time distribution (RTD), and exit mold types significantly influence the extrusion process. Additionally, downstream processes like cooling, grinding, and coating also impact the stability and bioavailability of the final product.
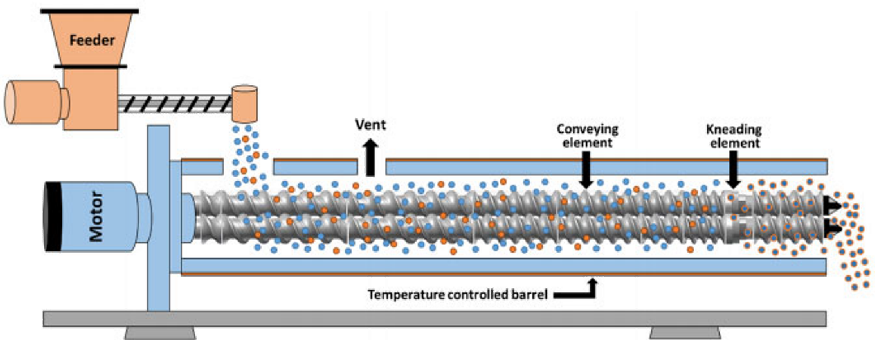
Hot melt extrusion process
HME produces solid formulations with uniform shape and size.
Uniform drug release from HME formulations ensures enhanced bioavailability.
Suitable for thermal stable drugs. Offering a continuous, highly reproducible production process and eliminating batch-to-batch variations.
Process Analytical Technology (PAT) can be used for real-time monitoring during production.
Applicable to large-scale industrial manufacturing.
Environmentally friendly, no solvent consumption.
HME operates at elevated temperatures, potentially leading to the decomposition and degradation of certain drugs.
HME necessitates the use of APIs at a minimum of kilogram-scale quantities.
Several Marketed Oral Formulations Employing Hot Melt Extrusion Technology
Product Name | Main Ingredient | FDA Approval Date | Manufacturer | Dosage Form |
Norvir | Ritonavir | 2010 | Abbott | Tablet |
Belsomra | Suvorexant | 2014 | Merck | Tablet |
Venclexta | Venetoclax | 2016 | Abbott | Tablet |
Lynparza | Olaparib | 2018 | AstraZeneca & Merck | Capsule/Tablet |
Braftovi | Encorafenib | 2018 | Pfizer | Tablet |
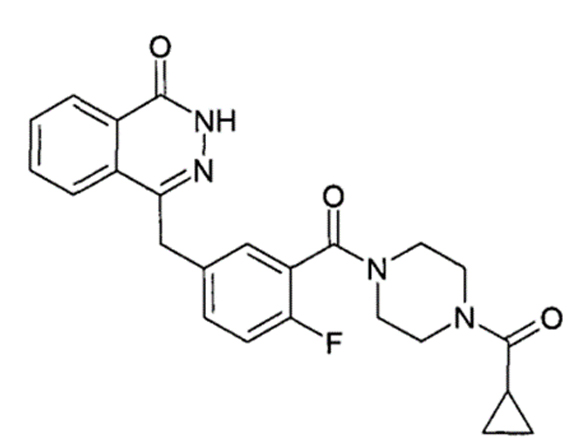
Olaparib Chemical Structure
Olaparib, the inaugural oral poly (ADP-ribose) polymerase (PARP) inhibitor approved by the FDA, operates by attacking DNA damage repair pathways, creating "synthetic lethality" to specifically target and destroy cancer cells while sparing healthy ones. It is authorized for various uses, including in ovarian, breast, prostate, and pancreatic cancers. Demonstrating remarkable therapeutic outcomes, Olaparib has been marketed in over 90 countries globally, achieving worldwide sales of $3.7 billion in 2022.
Although Olaparib has emerged as a major success in oncology, it encountered significant hurdles during its early development stages, mainly due to its poor bioavailability. Initial research focused on different formulations and strategies to effectively launch the drug in the market.
Olaparib, categorized as a BCS class IV drug, is a weakly acidic molecule with nearly neutral properties in the physiological pH range. It has an equilibrium solubility of about 0.1 mg/mL in aqueous buffers and its solubility in simulated gastrointestinal fluids varies between 0.12 to 0.20 mg/mL. The maximum solubility observed in Fed State Simulated Intestinal Fluid (FeSSIF) is only 0.20 mg/mL. Due to its low solubility and permeability, Olaparib has limited bioavailability, which restricts its clinical application.
In the early stages of development to tackle these challenges, the innovator investigated tablet and capsule formulations using a semi-solid triglyceride of lauric acid and glycerol (LMG). In preclinical studies in canines, the capsule formulation exhibited superior bioavailability. The Initial formulation development focused on two approaches to enhance the bioavailability of this poorly soluble compound: a solution in a capsule and a crystalline solid dispersion in a capsule. However, due to the lack of suitable solvent systems and concerns about issues like leakage, stability, and interactions between the excipients and the active ingredient in liquid-filled capsules, the focus shifted to developing crystalline solid dispersion capsules. To increase the drug's surface area exposure, the innovator utilized a micronization approach for active pharmaceutical ingredients (API). Despite the optimization of the formulation, the proportion of API in the formulation remained comparatively low.
Given that the dosage regimen of the capsule product was suboptimal, requiring patients to consume eight capsules at once (each 50 mg, twice daily, amounting to 400 mg per intake), the innovator sought to reduce the patient’s treatment load. The drug formulation was revisited, initially trying to create standard immediate-release tablets using micronized API. However, despite using micronized API, the dissolution rate of this type of dosage form remained limited.
Since Olaparib has low solubility in aqueous media (≤0.1 mg/mL), the focus of its formulation development was on improving solubility. After comprehensive evaluations of various advanced formulations, it was discovered that Olaparib’s amorphous solid dispersion prepared by hot-melt extrusion (HME) significantly improved bioavailability compared to other formulations (i.e., using glycerol monolaurate (LMG) capsule formulations). The amorphous solid dispersion, combining the active ingredient with a polymer carrier, not only increased the drug load in a single dosage unit (150 mg per tablet), but also lessened the dosage frequency (adjusted 300 mg twice daily, equating to two tablets). Additionally, it enhanced the drug solubility and bioavailability. Consequently, the daily active ingredient dose in the tablet form was lower than in the capsule. The exposure of 300 mg tablet was comparable to the original 400 mg marketed capsule, which contained a considerable amount of surfactant. This substantially improved patient compliance and marked a significant enhancement of the product.
The core team of Crystal Formulation Services (a subsidiary of Crystal Pharmatech) boasts expertise in polymer materials, chemical engineering, and mechanics. With a foundation in rheology and engineering principles, Crystal Formulation Services has gained substantial experience in the field of HME, covering both immediate and sustained release formulations, and possesses considerable knowledge in process scale-up and commercial manufacturing. Equipped with top-notch facilities for rheological research, formulation screening, and from small-scale trials to commercial production process equipment and analytical instruments. Committed to aiding innovative pharmaceutical companies, Crystal Formulation Services specializes in addressing the challenges associated with poorly soluble drugs through the application of the HME technique.
1. Patent CN102238945B;
2. EMA public assessment report;