Technical Article
Predicting Physical Stability of Amorphous Solid Dispersions (ASD)
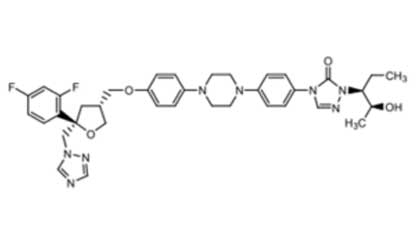
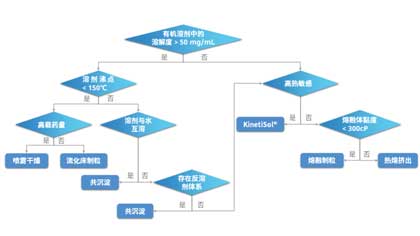
01 IntroductionAt present, new drug compounds are becoming increasingly complex, and the proportion of poorly water-soluble drugs continues to rise. It is estimated that 40% of marketed oral drugs and...










.webp)

.webp)