News
13 Nov 2024
Improving the Manufacture of mRNA Biologics
13 Nov 2024
 Mol2Med™ Integrated Services
Crystal Pharmatech are a specialized CRO/CDMO focused on Solid State Research, Pre-Formulation, Formulation Development and Manufacturing. Our strength is our focus and expertise in these specialties...
Mol2Med™ Integrated Services
Crystal Pharmatech are a specialized CRO/CDMO focused on Solid State Research, Pre-Formulation, Formulation Development and Manufacturing. Our strength is our focus and expertise in these specialties...
 Formulation Development
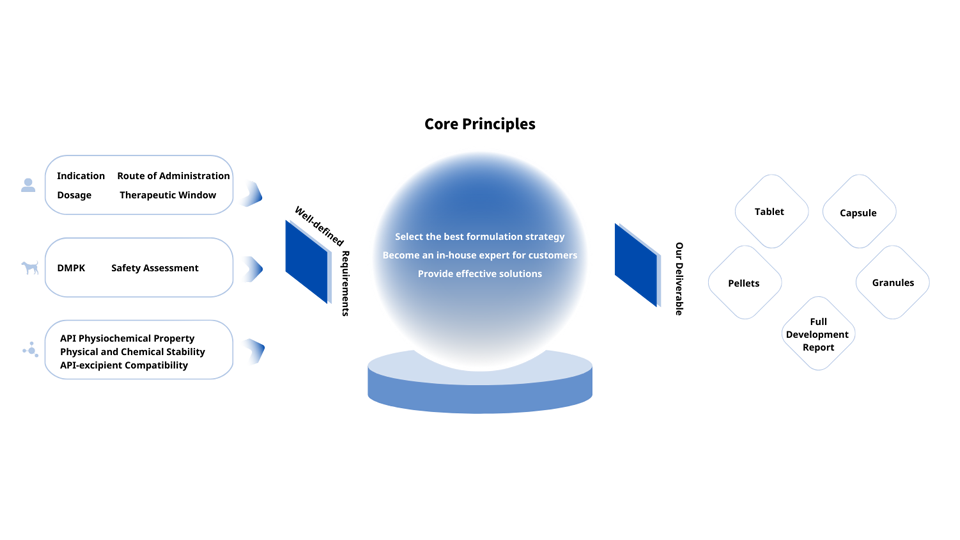
We guide our customers into making smarter formulation choices. Ultimately, this reduces the risk of clinical failure and ensures your product reaches the clinic rapidly via the most efficient route.
Formulation Development
We guide our customers into making smarter formulation choices. Ultimately, this reduces the risk of clinical failure and ensures your product reaches the clinic rapidly via the most efficient route.
 Solid Form Screening and Selection
Solid form is a general term that refers to both crystalline and amorphous materials. The solid form will impact active pharmaceutical ingredient (API) development properties such as solubility, dissolution rate, stability, hygroscopicity, and bioavailability.
Solid Form Screening and Selection
Solid form is a general term that refers to both crystalline and amorphous materials. The solid form will impact active pharmaceutical ingredient (API) development properties such as solubility, dissolution rate, stability, hygroscopicity, and bioavailability.
 Solid-State Characterization/FAST
Our Solid State Characterization Service, which is referred to as Focused Analytical Solids Testing (FAST), covers many routine analyses encountered during pharmaceutical development. The FAST concept is directly aligned with our goal of partnering with organizations instead of merely providing data.
Solid-State Characterization/FAST
Our Solid State Characterization Service, which is referred to as Focused Analytical Solids Testing (FAST), covers many routine analyses encountered during pharmaceutical development. The FAST concept is directly aligned with our goal of partnering with organizations instead of merely providing data.
 Oral Solid Dosage Forms
Most small molecule drugs are formulated as oral solid dosage (OSD) forms, such as tablets or capsules. OSD has been an important part of the global pharmaceutical market. The formulation team of Crys...
Oral Solid Dosage Forms
Most small molecule drugs are formulated as oral solid dosage (OSD) forms, such as tablets or capsules. OSD has been an important part of the global pharmaceutical market. The formulation team of Crys...
 Bioanalytical and Biomarker Services
With more than l00 years of combined industry experience of our leadership team and three GLP compliant laboratories, we are ready for all your current and emerging bioanalytical needs.
Bioanalytical and Biomarker Services
With more than l00 years of combined industry experience of our leadership team and three GLP compliant laboratories, we are ready for all your current and emerging bioanalytical needs.
 Single Crystal Growth & Structure Determination
Single crystal growth and structure determination technology are the main methods to determine the absolute configuration of drug molecules and identify the crystal forms absolutely. The single crysta...
Single Crystal Growth & Structure Determination
Single crystal growth and structure determination technology are the main methods to determine the absolute configuration of drug molecules and identify the crystal forms absolutely. The single crysta...
 pKa/LogP/LogD Measurements
Earlier than ever before in the development timeline, biotech can achieve a comprehensive understanding of their molecule's physicochemical properties. Using material-sparing automation, Crystal P...
pKa/LogP/LogD Measurements
Earlier than ever before in the development timeline, biotech can achieve a comprehensive understanding of their molecule's physicochemical properties. Using material-sparing automation, Crystal P...
 Amorphous Solid Dispersion (Spray Drying & Hot-melt Extrusion)
Recent statistics indicate that approximately 70% of small molecules under development belong to Biopharmaceutics Classification System (BCS) II or IV poorly water-soluble drugs. At Crystal Pharmatech...
Amorphous Solid Dispersion (Spray Drying & Hot-melt Extrusion)
Recent statistics indicate that approximately 70% of small molecules under development belong to Biopharmaceutics Classification System (BCS) II or IV poorly water-soluble drugs. At Crystal Pharmatech...
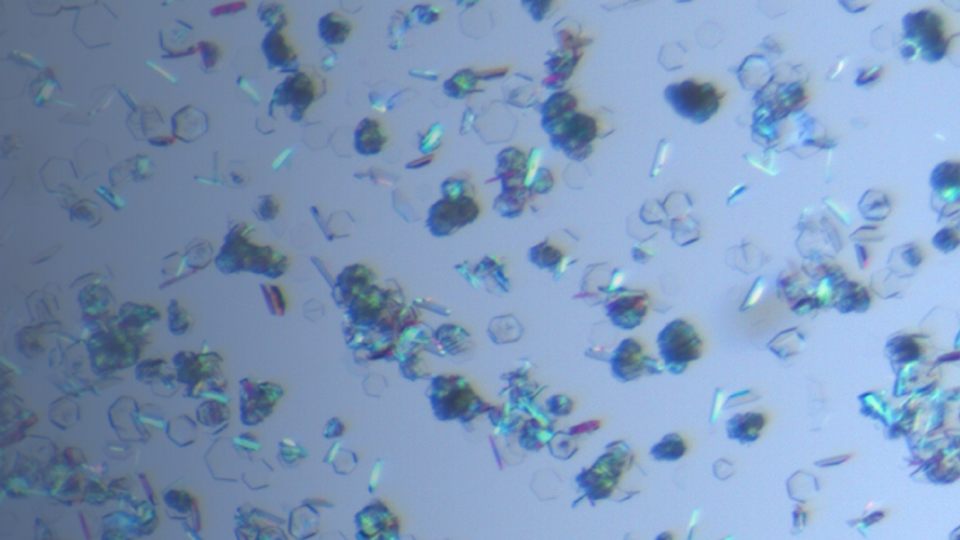 Crystallization Development
The aim of a crystallization process development is to find a crystallization process which can stable produce the optimal solid form with all desired properties such as particle size, particle shape,...
Crystallization Development
The aim of a crystallization process development is to find a crystallization process which can stable produce the optimal solid form with all desired properties such as particle size, particle shape,...
 Stability and Solubility Studies
Chemical and physical stability. The intent of the stability study is to evaluate the chemical and physical stability of the API in solution and solid-state. The solid-state evaluation will probe the pr...
Stability and Solubility Studies
Chemical and physical stability. The intent of the stability study is to evaluate the chemical and physical stability of the API in solution and solid-state. The solid-state evaluation will probe the pr...
 Comprehensive Physicochemical Property Evaluation
A comprehensive physicochemical property evaluation is an important premise for the development of formulation and process. It provides an important basis for the druggability evaluation of the compou...
Comprehensive Physicochemical Property Evaluation
A comprehensive physicochemical property evaluation is an important premise for the development of formulation and process. It provides an important basis for the druggability evaluation of the compou...
 Pediatric Formulation (Mini-tablet)
The development of acceptable, palatable pediatric formulations is undergoing transformation within the industry today, driven by patient needs and regulatory requirements. Therefore, the demand for a...
Pediatric Formulation (Mini-tablet)
The development of acceptable, palatable pediatric formulations is undergoing transformation within the industry today, driven by patient needs and regulatory requirements. Therefore, the demand for a...
 Chiral Separation
More than 70% of drug candidates worldwide are chiral. Typically, for chiral API, only one enantiomer or diasteromer is biologically active or desirable. Therefore, the production of enantiopure compounds or diastereomers is imperative. In the production of small molecule drugs with desired chirality, separation via crystallization can be much more economical and environmentally favorable than chromatography.
Chiral Separation
More than 70% of drug candidates worldwide are chiral. Typically, for chiral API, only one enantiomer or diasteromer is biologically active or desirable. Therefore, the production of enantiopure compounds or diastereomers is imperative. In the production of small molecule drugs with desired chirality, separation via crystallization can be much more economical and environmentally favorable than chromatography.
 In-Silico PBPK Modeling and Simulation
Crystal Pharmatech now provides Physiologically Based Pharmacokinetics (PBPK) simulation capability.
In-Silico PBPK Modeling and Simulation
Crystal Pharmatech now provides Physiologically Based Pharmacokinetics (PBPK) simulation capability.