News
13 Nov 2024
Improving the Manufacture of mRNA Biologics
13 Nov 2024
 Developability Assessment
For lead compounds or candidate compounds, Crystal pharmatech can make an objective evaluation of the development potential and risks of drugs through comprehensive and accurate evaluation of the physicochemical properties of compounds, combined with a variety of mature development evaluation models and GastroPlus® software.
Developability Assessment
For lead compounds or candidate compounds, Crystal pharmatech can make an objective evaluation of the development potential and risks of drugs through comprehensive and accurate evaluation of the physicochemical properties of compounds, combined with a variety of mature development evaluation models and GastroPlus® software.
 Development and Production Application Cases of Amorphous Solid Dispersion Formulations (II) - Spray Drying
In the preceding article, we discussed the application of hot-melt extrusion technology in developing amorphous solid dispersions (ASD) for pharmaceutical formulations. The process involves dispersing...
Development and Production Application Cases of Amorphous Solid Dispersion Formulations (II) - Spray Drying
In the preceding article, we discussed the application of hot-melt extrusion technology in developing amorphous solid dispersions (ASD) for pharmaceutical formulations. The process involves dispersing...
 CATUG and Crystal Bio Establish Strategic Partnership, Launching “CATUG-Crystal” Joint Lab Dedicated to Advanced Nucleic Acid Analytical Services
CAMBRIDGE, Massachusetts & CRANBURY, New Jersey, February 21, 2024, – CATUG Inc. (CATUG) and Crystal Bio, a member of Crystal Pharmatech, announced today a long-term strategic partnership to prov...
CATUG and Crystal Bio Establish Strategic Partnership, Launching “CATUG-Crystal” Joint Lab Dedicated to Advanced Nucleic Acid Analytical Services
CAMBRIDGE, Massachusetts & CRANBURY, New Jersey, February 21, 2024, – CATUG Inc. (CATUG) and Crystal Bio, a member of Crystal Pharmatech, announced today a long-term strategic partnership to prov...
 Utilizing Compaction Simulation for Advancing Oral Dosage Formulation Design
Utilizing Compaction Simulation for Advancing Oral Dosage Formulation Design
 The 16th Drug Discovery Strategic Summit (DDSS) - San Francisco
Date: October 9-10, 2024Location: San Francisco Airport Marriott WaterfrontBooth: 3Attendee: Robert Wenslow, Ph.D. (Speaker) Ridwan Islam, Ph.D.Presentation title: “The Best Use of 10 mg” - Achieve ...
The 16th Drug Discovery Strategic Summit (DDSS) - San Francisco
Date: October 9-10, 2024Location: San Francisco Airport Marriott WaterfrontBooth: 3Attendee: Robert Wenslow, Ph.D. (Speaker) Ridwan Islam, Ph.D.Presentation title: “The Best Use of 10 mg” - Achieve ...
 Bioanalytical and Biomarker Services
With more than l00 years of combined industry experience of our leadership team and three GLP compliant laboratories, we are ready for all your current and emerging bioanalytical needs.
Bioanalytical and Biomarker Services
With more than l00 years of combined industry experience of our leadership team and three GLP compliant laboratories, we are ready for all your current and emerging bioanalytical needs.
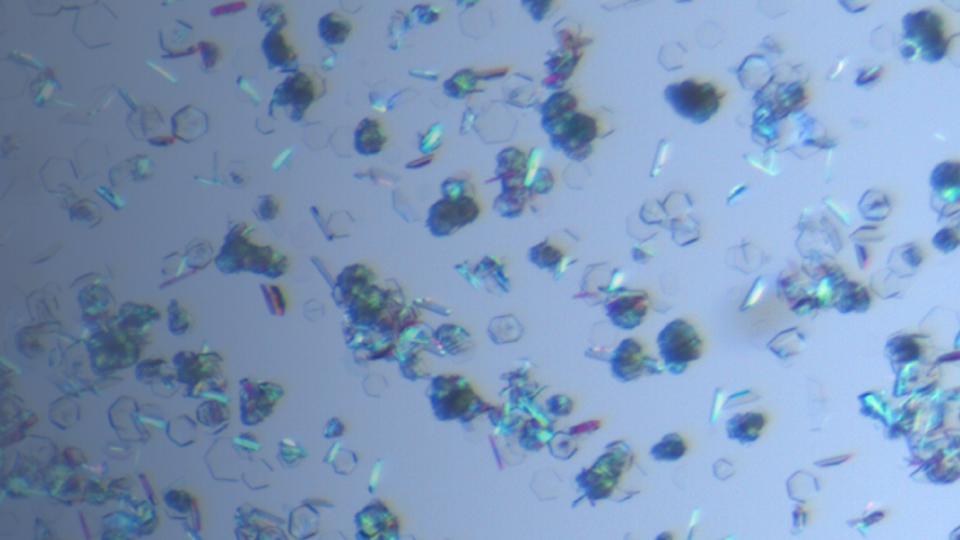 Single Crystal Growth & Structure Determination
Single crystal growth and structure determination technology are the main methods to determine the absolute configuration of drug molecules and identify the crystal forms absolutely. The single crysta...
Single Crystal Growth & Structure Determination
Single crystal growth and structure determination technology are the main methods to determine the absolute configuration of drug molecules and identify the crystal forms absolutely. The single crysta...
 Bioavailability Enhancement for Insoluble Compounds & PROTAC & Oral Peptides
With the ever-increasing demand for the absorbability of small-molecule oral innovative drugs, more and more new molecules have hydrophobic properties, resulting in very low solubility. According to the latest statistics, about 70% of the small-molecule drugs under research are insoluble substances of BCS II or IV.
Bioavailability Enhancement for Insoluble Compounds & PROTAC & Oral Peptides
With the ever-increasing demand for the absorbability of small-molecule oral innovative drugs, more and more new molecules have hydrophobic properties, resulting in very low solubility. According to the latest statistics, about 70% of the small-molecule drugs under research are insoluble substances of BCS II or IV.
 Events
Events